
Emerging technologies promise to improve clinical trial efficiency across the board – but whether or not the industry will embrace them remains to be seen.
Recently, I’ve been reading a great deal about innovative new technologies and their potential impact on clinical trial efficiency. I just came across a great piece on the Partnerships in Clinical Trials blog that included some illuminating statistics about the ongoing struggle to keep costs under control.
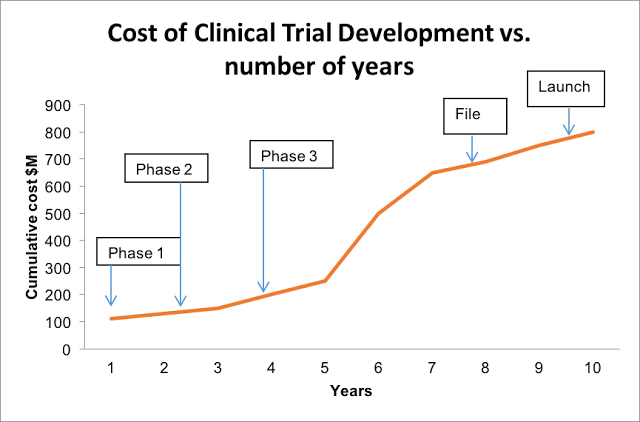
According to a few recent FDA reports, about 30% of drugs clear Phase I trials, 14% clear Phase II trials, and a mere 9% make it through Phase III trials. Moreover, the approval process can take up to 11 years, which doesn’t even include the pre-clinical research phase.
(Source: Hype or hope? Can mHealth Accelerate Completion in Clinical Trials?)
As Sarah Iqbal, the article’s author, aptly points out, back in 1975, sponsors spent approximately $100 million annually (in today’s dollars) on R&D for drugs that eventually secured FDA approval. By 1987, that number shot up to $300 million, then to $1.3 billion by 2005. While the average price tag on FDA approval today is somewhat hard to nail down, it’s safe to assume that it’s continued on its upward tract.
In fact, Forbes contributor Matthew Herper tallied R&D spending from 12 leading Pharma companies between 1997 to 2011, which amounted to a whopping $802 billion for 139 new drugs – that’s $5.8 billion per drug.
As the number of clinical studies for new drugs and medical devices has increased year-over-year (up nearly 4057% since 2000), proving that a new product is superior to an existing one has become increasingly difficult. Moreover, regulatory trends have made running clinical trials more complex, with a greater emphasis placed on site/patient monitoring and hard data.
Clearly, the industry needs to change if it hopes to reverse this pattern of skyrocketing R&D costs and diminishing profit margins, while also improving outcomes for patients and reducing the overall cost of care. Here are a few emerging tactics and technologies that might be able to help.
1. Tracking Technologies
Patient recruitment and retention is the number one contributor to delays in trial completion – in fact, approximately 29% of a trial’s total time and budget can be attributed to the enrollment process. One of the biggest mistakes that sponsors make is to not track recruitment efforts. By monitoring a variety of key metrics, such as digital ad performance, investigator site lead response time, and patient retention rates, sponsors will be able to make data-led decisions when it comes to strategy and budget allocation. Moreover, having a clear sense of what worked and what didn’t will help improve outcomes in future trials.
2. mHealth
Mobile health, or “mHealth,” technologies are rapidly gaining traction as an incredibly cost-effective and participant-friendly way to enhance data collection efforts, bolster patient engagement, and streamline the overall clinical trial process. Already, major Pharma companies including Pfizer, Astrazeneca, and GlaxoSmithKline are investing in mHealth pilot programs that extend across the entire development process. As the importance of data transparency and patient centricity continues to grow, it’s likely that mobile health technologies will play an increasingly integral role in clinical trials in the future.
3. Wearables
According to Iqbal, a growing number of clinical trials are now leveraging wearable technologies in order to “align investigator site and patient needs with faster study execution and reduced costs.” According to Applied Clinical Trials, major tech players have already recognized the potential impact of these technologies – Google recently developed a smart wristband for clinical research that can measure pulse, heart rhythm, skin temperature, light exposure, and noise levels; Apple’s new ResearchKit enables researchers to develop apps that manage data collected by wearables and smartphones during clinical studies.
There are even devices that can measure drug adherence. For instance, ingestible monitors can track data on drug dosage, dose timing, and a variety of physiologic responses, then transmit that information in real-time to the patient’s mobile device.
The point is, the industry has been fighting a losing battle against surging costs, trial efficiency, and approval rates for years, largely because of its unwillingness to adapt. But now, a wealth of new and exciting technologies are emerging that promise to address many of these issues head-on. Sponsors, CROs, and sites need to embrace change and innovation if they hope to remain profitable and improve outcomes for patients now and going forward.




