Behind the screens: Meet Rolivhuwa, driving trial efficiency from day one

In our Behind the Screens series we are spotlighting our Site Companions: the dedicated site engagement specialists, who power site support and clinical trial efficiency.
Behind the screens: Meet Merusha, leading with empathy to ease site burden

In this Behind the Screens spotlight, we feature Merusha Naidoo, a site team leader whose relationship-driven approach helps ease burden and boost performance across trial sites around the world.
Accelerating patient enrollment with Full-Site Support

9.7x faster patient progressions saves $754,000 and dramatically reduces site burden
Transforming dermatology trial recruitment

How SubjectWell’s strategic digital marketing dramatically reduced costs and boosted patient enrollment
Maximizing trial recruitment for peanut allergies

How SubjectWell’s strategic digital marketing dramatically reduced costs and boosted patient enrollment
Accelerating patient-centric clinical trials through science-based planning, delivery and support

Join experts from Subjectwell and Veristat in this virtual roundtable as they discuss proven approaches for navigating the complexities of modern clinical trials.
Addressing stigma and logistical hurdles in psychiatric trials
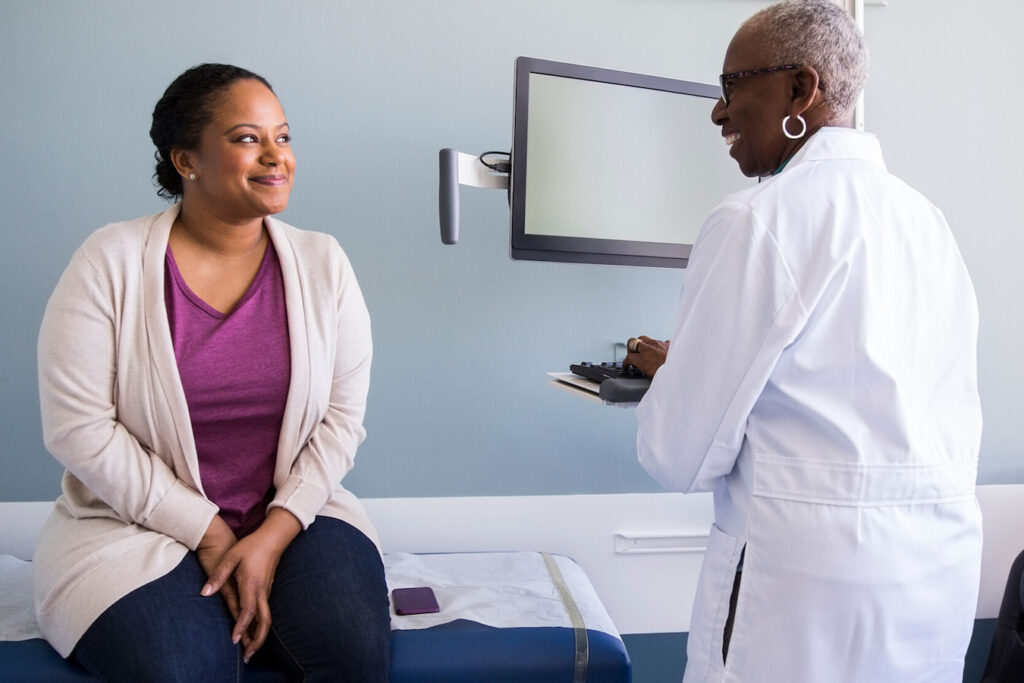
Learn how SubjectWell tackles key barriers in psychiatric trials—like stigma, documentation delays, and caregiver burden—with services that improve engagement, retention, and site efficiency.
Not all patients are the same: Why GLP-1 trials demand tailored engagement

Not every GLP-1 trial participant sees their condition in the same way. From diabetes to obesity and addiction, patient mindsets influence trial participation. Learn why tailored engagement strategies are essential for successful GLP-1 recruitment.
Meet Mbali: Guiding patients with care, clarity, and cultural understanding

Learn about a day in the life of a Patient Companion at SubjectWell and how they support patients throughout their clinical trial journey.