Background
A top 10 pharmaceutical company partnered with SubjectWell to provide a global patient recruitment program for its rare disease, phase III Autosomal Dominant Polycystic Kidney Disease (ADPKD) clinical study. SubjectWell designed a digital patient recruitment solution, providing localized outreach in multiple countries, addressing complex global data privacy requirements, and ultimately driving high impact enrollment results
The challenge
- Enroll a significant number of patients in a low prevalence condition (42.6 per 100,000 population)
- Provide localized outreach in multiple countries
- Address complex global data privacy requirements
Action
STRATEGY
SubjectWell worked with the sponsor to determine which countries would produce high impact enrollment results, based on:
- Previous experience with successful programs in country
- Existing relationships with local partners (e.g. in both Europe and Japan)
- Ability to meet key data privacy regulations and SubjectWell’s certifications and compliance programs; APEC Processor, GDPR, EU/US PrivacyShield
- Ability to leverage digital recruitment investment across a large population (e.g. adding Austro-Bavarian population of ~7 million to German program)
TACTICS
SubjectWell developed a multi-channel digital outreach strategy for US, Japan, Germany, and Austria in native languages, composed of:
- Creative: Website, online pre-screener, and ads
- Digital outreach: Search, social, society outreach (PKD Foundation, National Kidney Foundation), and SubjectWell’s Direct Connect
- Personnel: Global call center, site recruitment support, and local country partners
- Technology: Compliant backend data capture under local data privacy laws and data distribution to the sites under regulatory guidelines
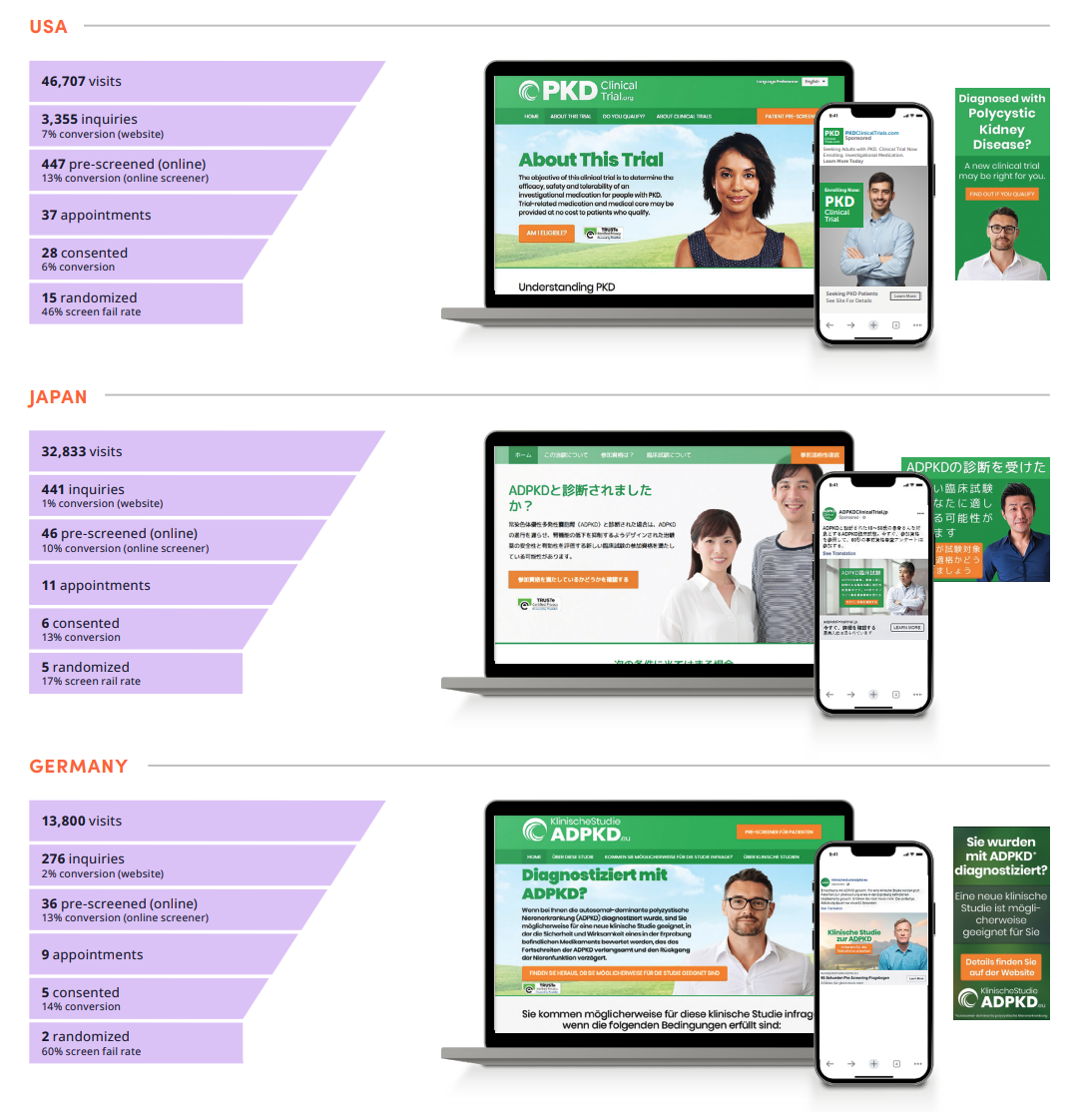
Results by country
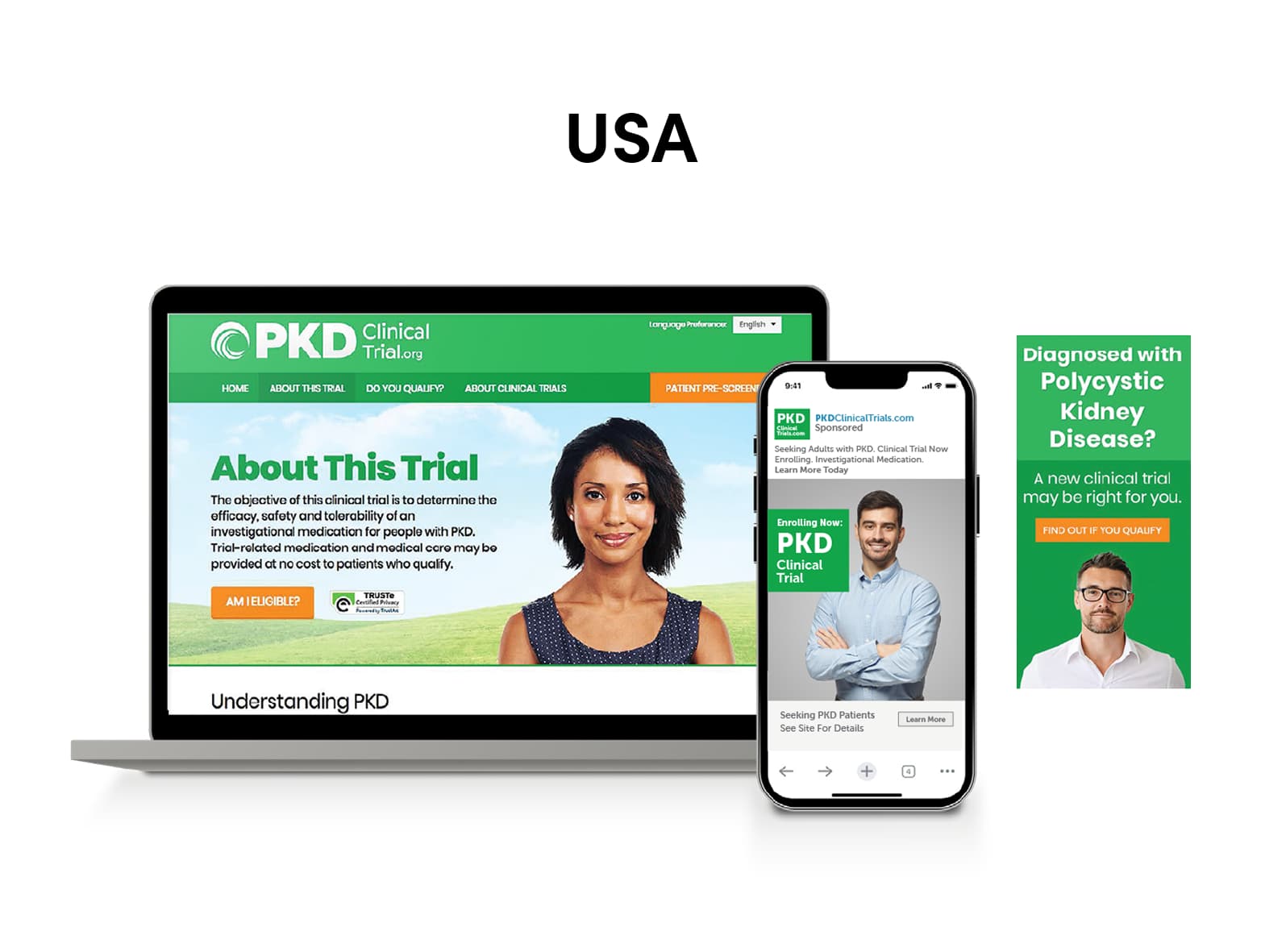
Results – USA
- 46,707 visits
- 3,355 inquiries
- 447 pre-screened (online)
- 37 appointments
- 28 consented
- 15 randomized

Results – Japan
- 32,833 visits
- 441 inquiries
- 46 pre-screened (online)
- 11 appointments
- 6 consented
- 5 randomized
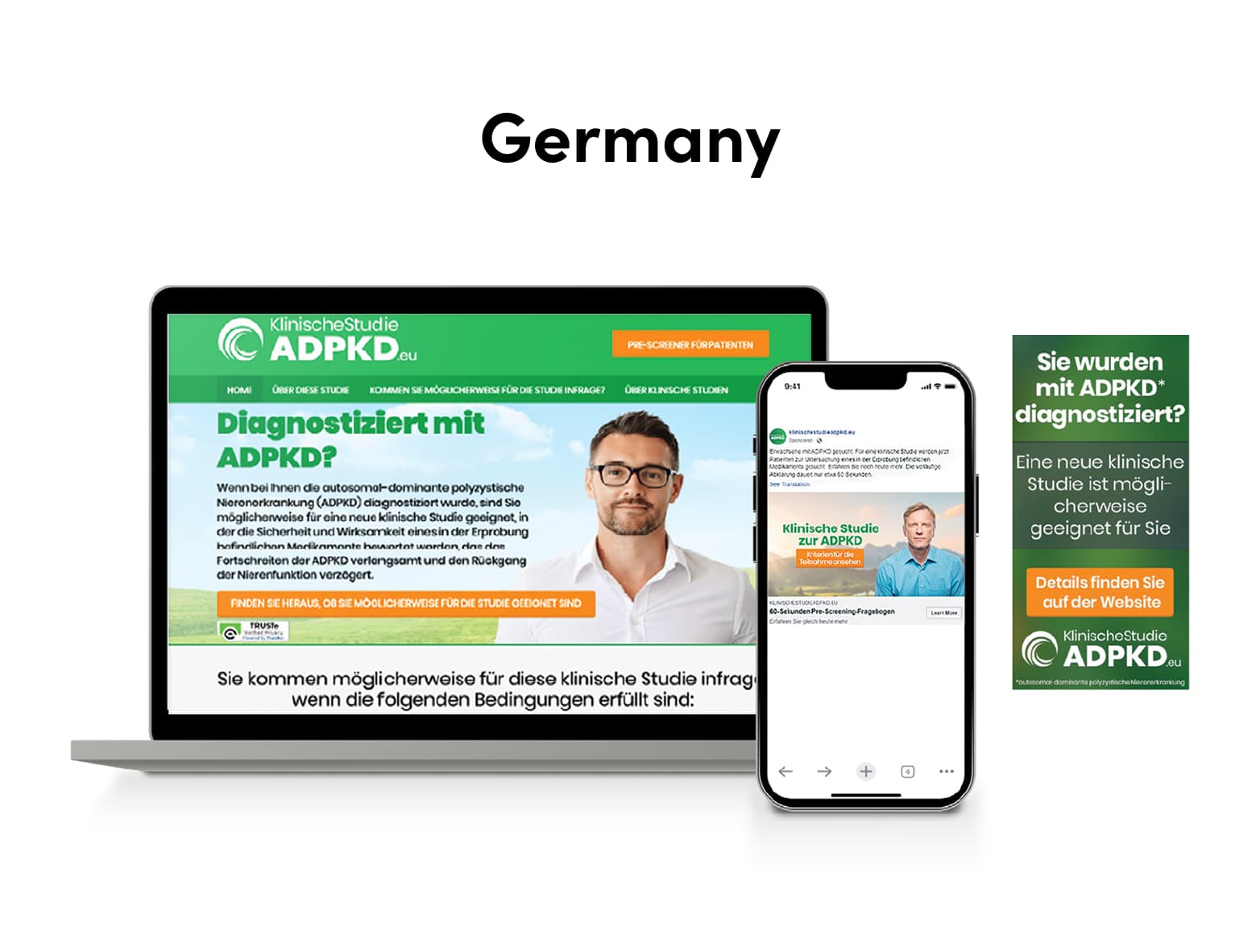
Results – Germany
- 13,800 visits
- 276 inquiries
- 36 pre-screened (online)
- 9 appointments
- 5 consented
- 2 randomized