Introducing the SubjectWell+ Platform
The SubjectWell+ Platform delivers the most comprehensive solutions for patient recruitment and site enablement, seamlessly paired with advanced commercial capabilities to connect patients with clinical trials and new treatments. Powered by a robust technology platform, a full suite of tailored services, creative expertise, and an extensive partner network, SubjectWell+ is backed by over 13 million motivated patients eager to participate in research that drives change.
It’s time for more. It’s time for SubjectWell+.
Innovative patient recruitment and commercial solutions
At SubjectWell, we collaborate with every key player in clinical research and commercial services to accelerate patient recruitment, improve site performance, optimize study outcomes, and drive market success. Whether you’re a sponsor, CRO, Site, Site Network, or Brand Marketers our tailored solutions meet your unique needs and deliver measurable results.
Explore how our specialized services empower your role in advancing healthcare breakthroughs and achieving commercial excellence.
It’s time to transform patient recruitment
The clinical trial landscape has changed dramatically. Protocols are more complex, patient needs have evolved, and regulatory standards are stricter. Globalization has made finding patients even more challenging. Yet, patient recruitment strategies have remained stagnant, leading to an 80% clinical trial failure rate and $40 billion in unrecoverable losses.
-
30%
Accelerate enrollment by
$40B
Recoup $40B in losses
13M+
patients in our network
-
47%
of patients identify as people of color
1,400+
studies completed
15,500+
sites served
-
100+
clinically trained patient companions
22
languages supported in our Companion Centers
Expertise across all therapeutic areas
-
AI/ML tools to reach the hardest-to-find patients
Seamless CTMS integrations
Real-time feedback for sponsors, sites, and CROs
Global recruitment for every Therapeutic Area
Rare, Complex, or Chronic Conditions
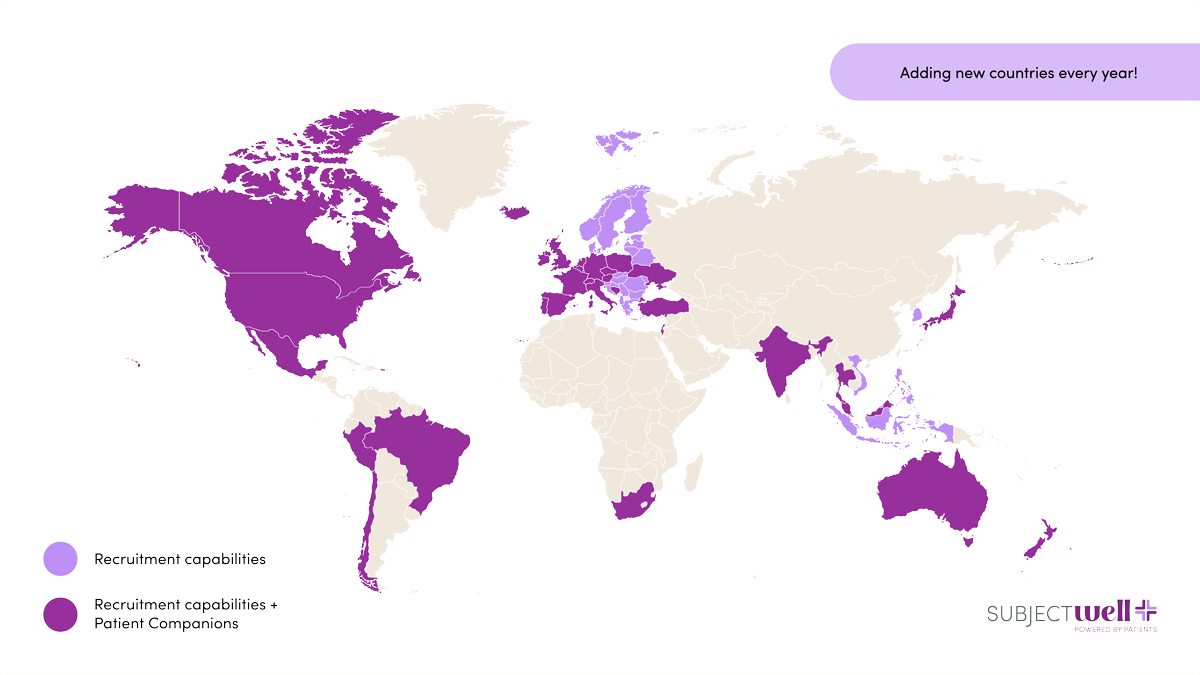
SubjectWell is your partner for patient recruitment across every therapeutic area, anywhere in the world. From rare disease and oncology to chronic conditions and vaccine studies, our expertise ensures your study’s success. With over 13 million patients in our marketplace, we’ve supported more than 500,000 patient referrals across 15,000 sites, delivering measurable results for sponsors and studies worldwide.
Building trust. Improving retention. Enhancing outcomes.
Clinical trials require trust and understanding as patients make significant sacrifices and navigate difficult decisions. At the same time, sites face increasing challenges such as administrative tasks, resource constraints, and data management burdens, all of which can detract from patient care and contribute to recruitment failures.
SubjectWell bridges these gaps with comprehensive solutions that ensure that patients stay informed, engaged, and valued throughout the recruitment and clinical development process while reducing both patient and site burden.
Insights from industry leaders
Stay informed with expert insights on clinical trial trends, patient recruitment strategies,
and healthcare innovations.
-

Behind the screens: Meet Merusha, leading with empathy to ease site burden
In this Behind the Screens spotlight, we feature Merusha Naidoo, a site team leader whose23 Jun 2025●2 min -

Beyond the Rainbow: Clinical research needs more than a month’s moment
SubjectWell CEO Fred Martin explores why symbolic support isn’t enough and how the steps the16 Jun 2025●4 min -

Addressing stigma and logistical hurdles in psychiatric trials
Learn how SubjectWell tackles key barriers in psychiatric trials—like stigma, documentation12 May 2025●3 min -

Not all patients are the same: Why GLP-1 trials demand tailored engagement
Not every GLP-1 trial participant sees their condition in the same way. From diabetes to obesity02 May 2025●3 min
Get started with SubjectWell
Join 3,000+ customers leveraging SubjectWell to accelerate clinical trial recruitment, engage patients, and drive study success. Let’s revolutionize research together.






