At SubjectWell, we continue to conduct surveys with patients to understand how their attitudes and feelings toward clinical trials have been altered in the wake of the pandemic. Having a grasp on these sentiments may now be more important than ever, as states continue the reopening process while positive cases continue to increase.
Our most recent survey was conducted in partnership with CISCRP, the Center for Information and Study on Clinical Research Participation. We worked with the organization to develop questions for patients, and then on the analysis of the data that was yielded.
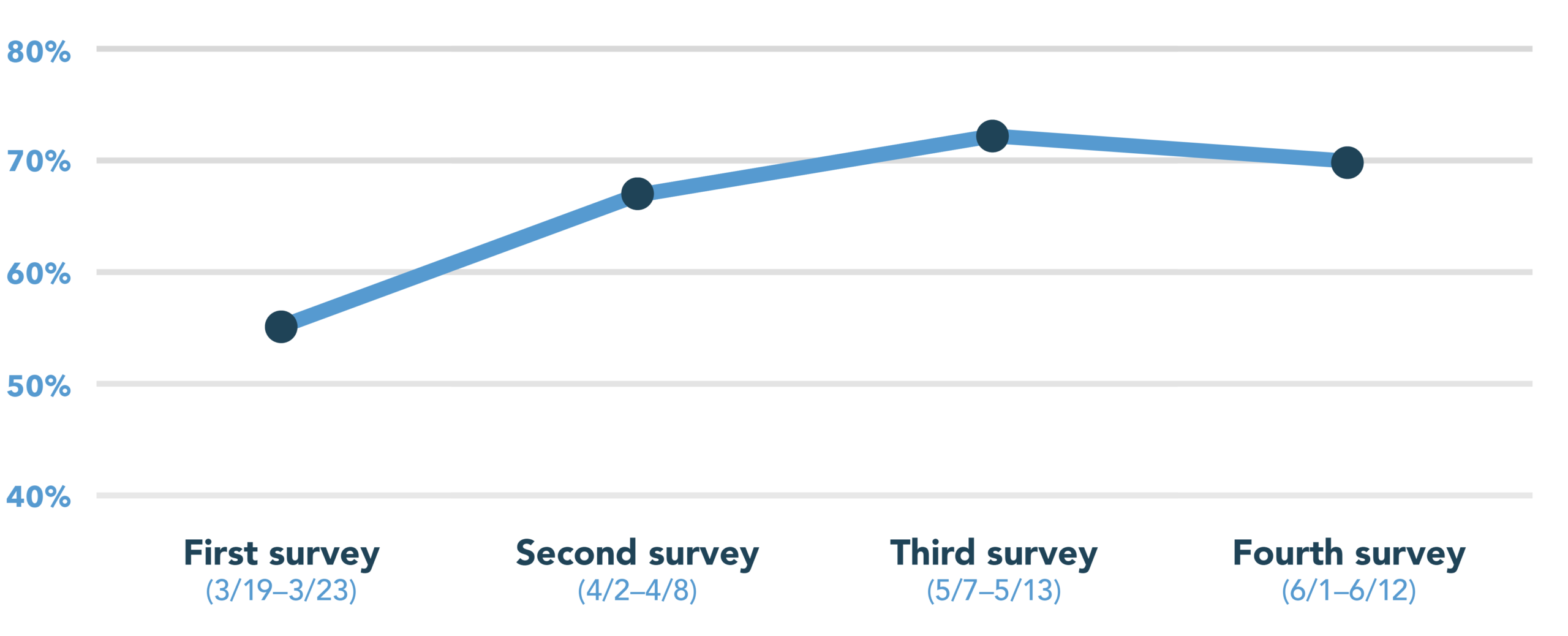
This fourth round of our survey was fielded between June 1 and 12, 2020. We polled 553 patients across the United States. This time around, we are taking a closer look at race and gender to better understand how these factors impact clinical trial participation.
As a refresher, you can read our reports on round one of our survey (conducted March 19–23) here, round two (conducted April 2–8) here, and round three (conducted May 7–13) here.
Here are some of the discoveries from our fourth round of research.
[scorr_infographic_wrap aos=”1″ align=”left”]
[scorr_infographic width=”75″]
Patients considering participation in a clinical trial
[/scorr_infographic]
[/scorr_infographic_wrap]
[scorr_infographic_wrap aos=”1″]
[scorr_infographic width=”100″]
[scorr_bar_chart_single percent=”84″]
84% feel it’s vital to know what to do if they contract COVID-19 while in a trial
[/scorr_infographic]
[scorr_infographic width=”100″]
[scorr_bar_chart_single percent=”73″]
73% want assurance that their health and safety will be protected at in-office visits
[/scorr_infographic]
[scorr_infographic width=”100″]
[scorr_bar_chart_single percent=”62″]
62% want the ability to communicate with a study doctor remotely
[/scorr_infographic]
[/scorr_infographic_wrap]
Differences by race.
As all clinical research is important, but studies specific to COVID-19 are obviously top of mind, we inquired how the current pandemic affects decision-making for non-COVID-19 studies.
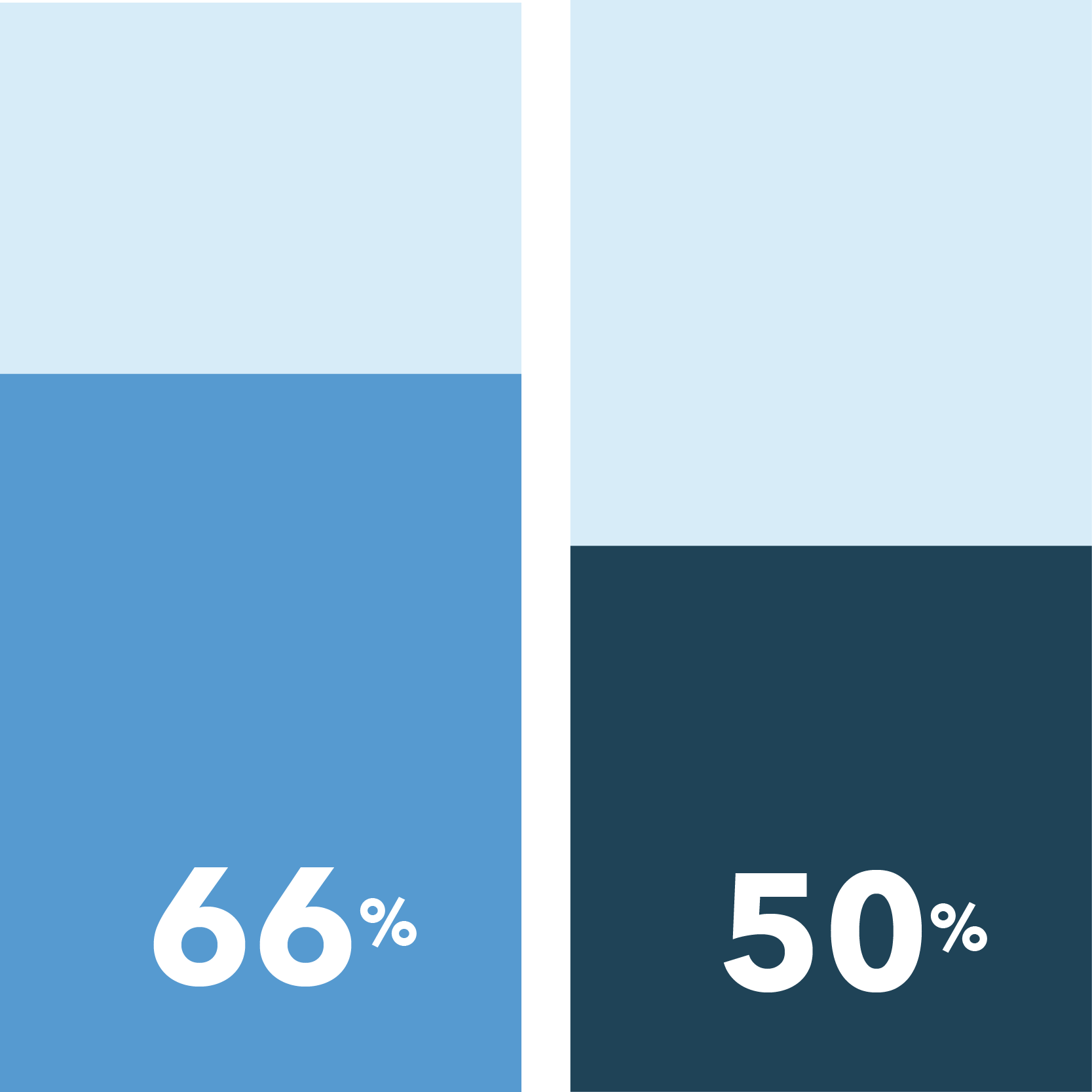
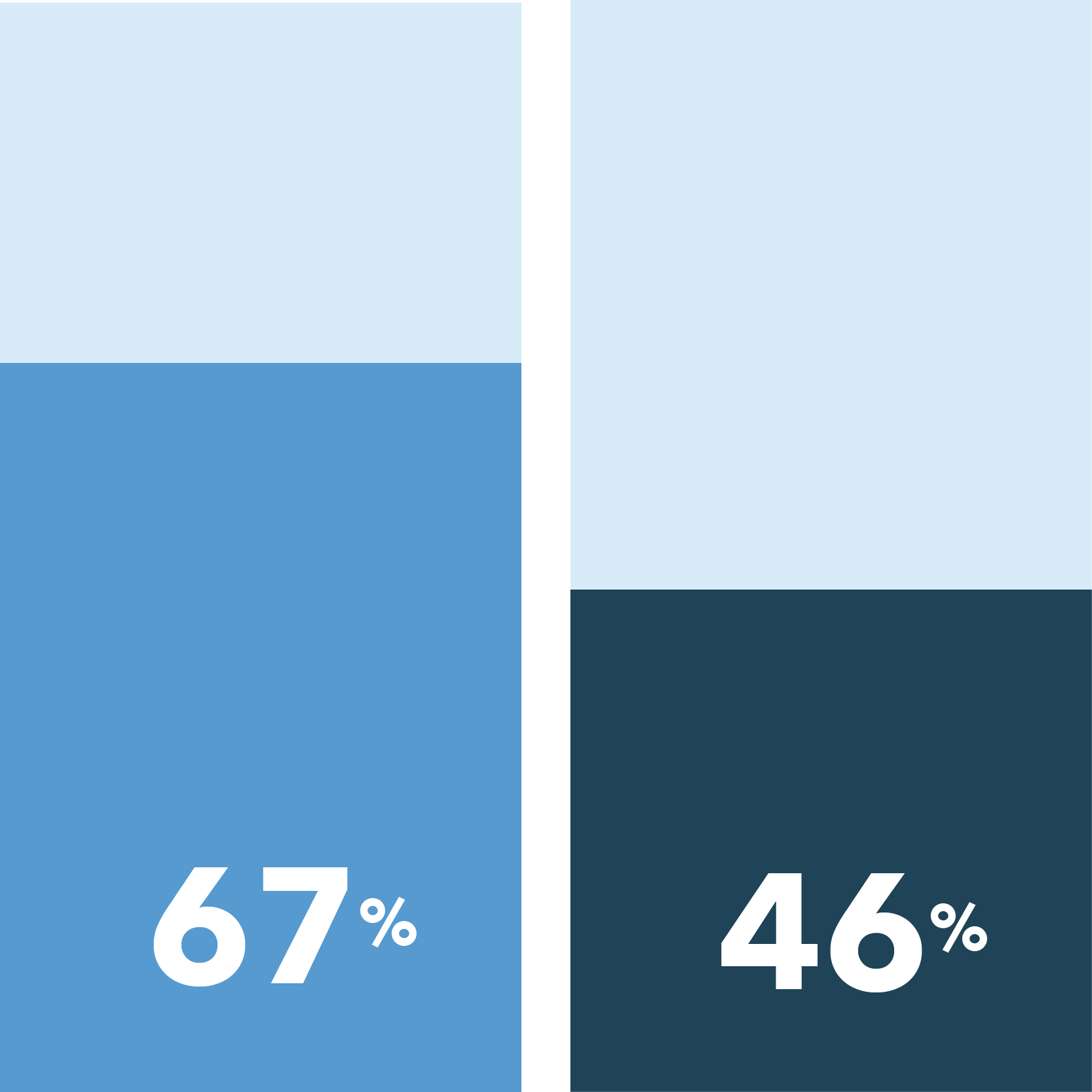
Overall, African American patients report more hesitations when considering participation in non-COVID-19 clinical trials and a greater desire for safety precautions if they were to participate compared to Caucasian patients.
- Far more African Americans than Caucasians responded that they are not at all likely to consider participation in a clinical trial for a medical condition other than COVID-19
- African Americans are more concerned than Caucasians about being exposed to COVID-19 if they were to enroll in a clinical trial
- African American patients are more concerned with precautionary measures like limiting the number of site visits and having the ability to send lab samples from home compared to Caucasian patients
Not at all likely to consider participating in a clinical trial for a condition other than COVID-19
[scorr_infographic_wrap aos=”1″]
[scorr_infographic width=”100″]
[scorr_bar_chart_single percent=”26″]
26% of African Americans
[/scorr_infographic]
[scorr_infographic width=”100″]
[scorr_bar_chart_single percent=”13″]
13% of Caucasians
[/scorr_infographic]
[/scorr_infographic_wrap]
Very concerned about COVID-19 exposure when taking part in a clinical research study
[scorr_infographic_wrap aos=”1″]
[scorr_infographic width=”100″]
[scorr_bar_chart_single percent=”56″]
56% of African Americans
[/scorr_infographic]
[scorr_infographic width=”100″]
[scorr_bar_chart_single percent=”31″]
31% of Caucasians
[/scorr_infographic]
[/scorr_infographic_wrap]
The following were identified as very important in easing COVID-19 concerns:
[scorr_infographic_key colors=”#649acb|#1f4357″ labels=”African Americans|Caucasians”]
[scorr_infographic_wrap aos=”1″]
[scorr_infographic width=”25″]
Concierge services
[/scorr_infographic]
[scorr_infographic width=”25″]
In-person visits
[/scorr_infographic]
[scorr_infographic width=”25″]
At-home lab collection
[/scorr_infographic]
[scorr_infographic width=”25″]
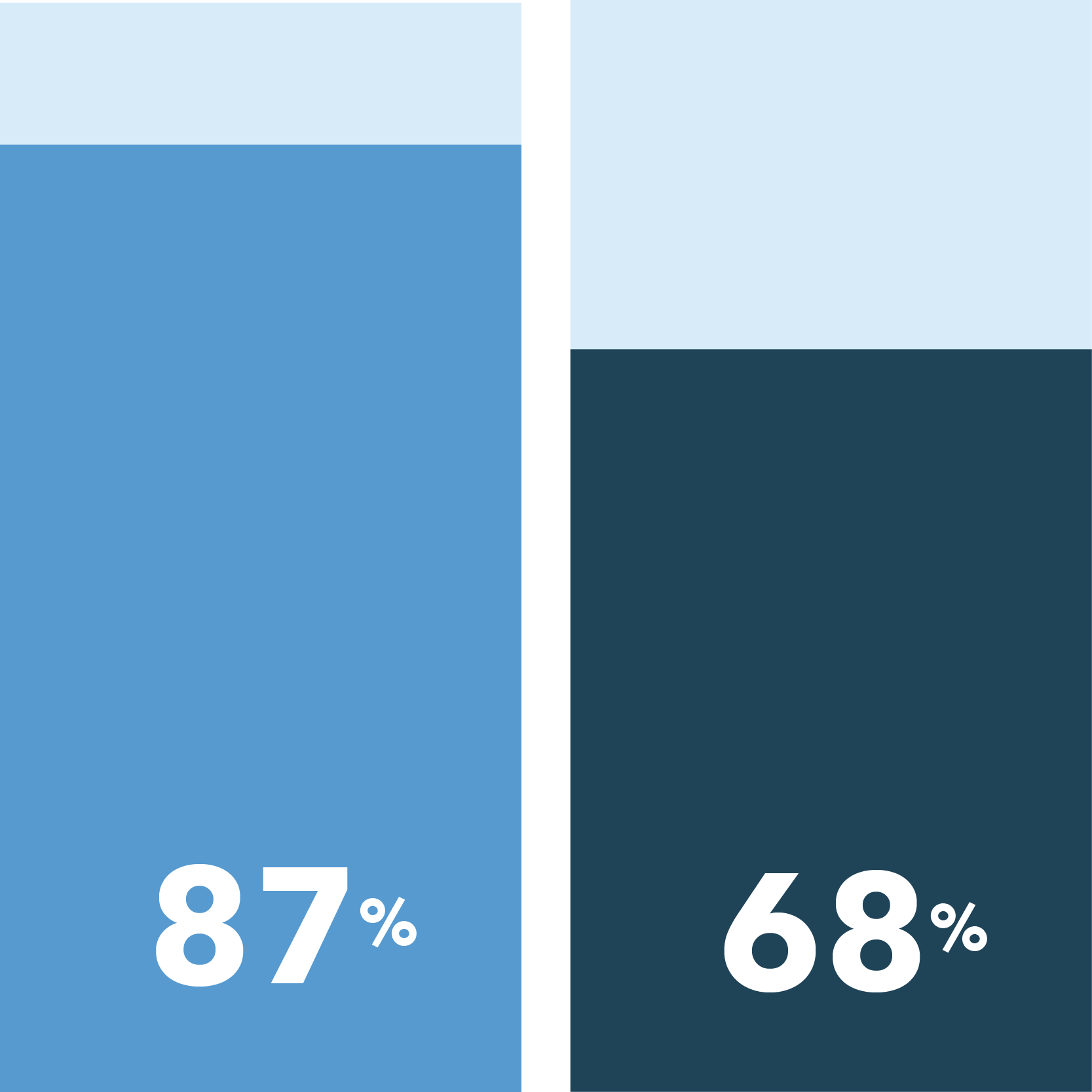
Health and safety protected at home
[/scorr_infographic]
[/scorr_infographic_wrap]
Differences by gender.
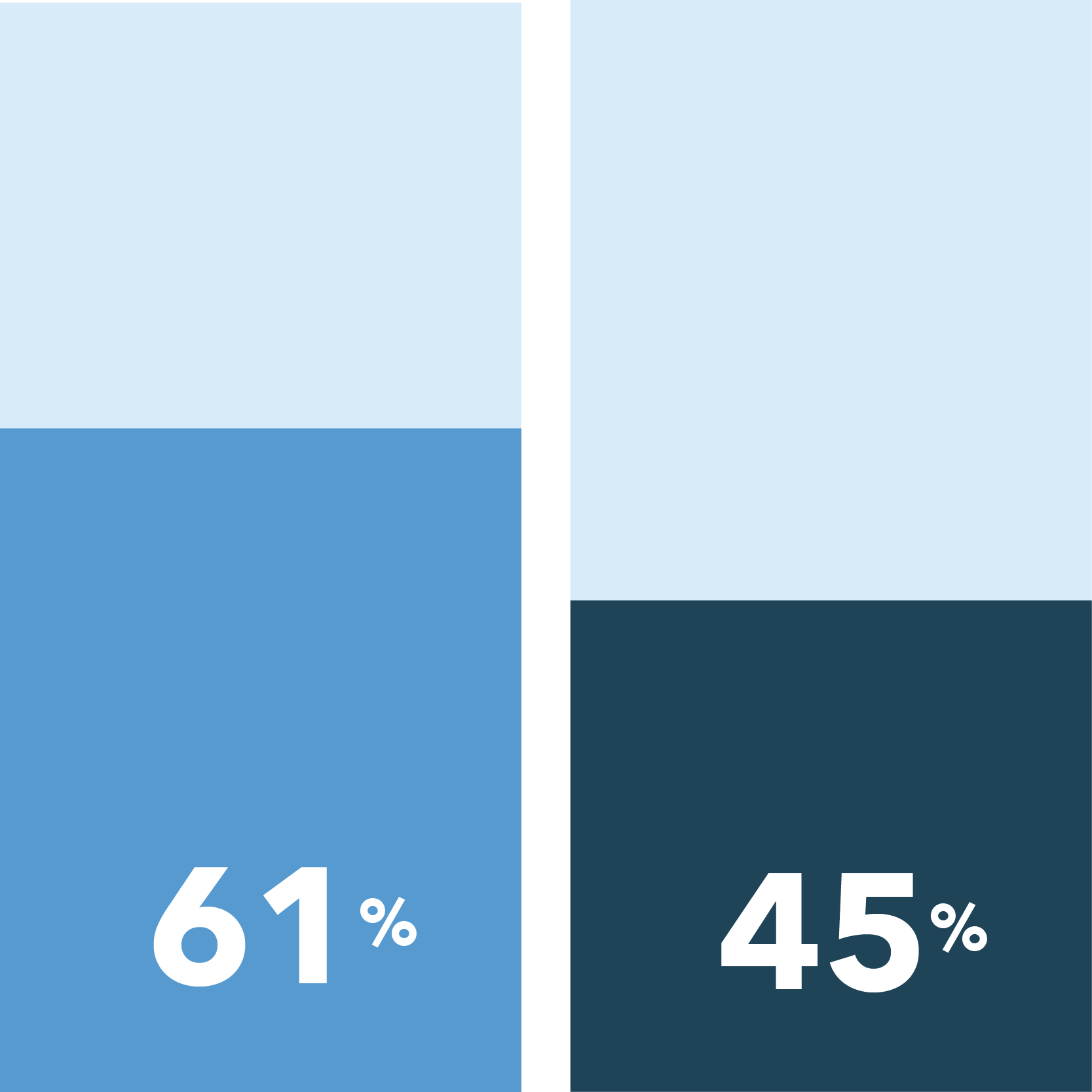
Our survey found that men are less hesitant to participate in non-COVID-19 clinical trials than women, and women have a greater need for safety precautions if they were to participate.
- Men were more likely to not be concerned at all about exposure to COVID-19 if they were to enroll in a clinical trial for a condition other than COVID-19
- Women place a higher value on precautions like being able to communicate with a study doctor remotely, having study medicine delivered to their home, and knowing their health and safety would be protected when visiting the study clinic
- Men do not feel that having a nurse come to their home for study visits is important
Not at all concerned about COVID-19 exposure when taking part in a clinical research study
[scorr_infographic_wrap aos=”1″]
[scorr_infographic width=”100″]
[scorr_bar_chart_single percent=”13″]
13% of Women
[/scorr_infographic]
[scorr_infographic width=”100″]
[scorr_bar_chart_single percent=”23″]
23% of Men
[/scorr_infographic]
[/scorr_infographic_wrap]
The following were identified as very important in easing COVID-19 concerns:
[scorr_infographic_key colors=”#649acb|#1f4357″ labels=”Women|Men”]
[scorr_infographic_wrap aos=”1″]
[scorr_infographic width=”33″]
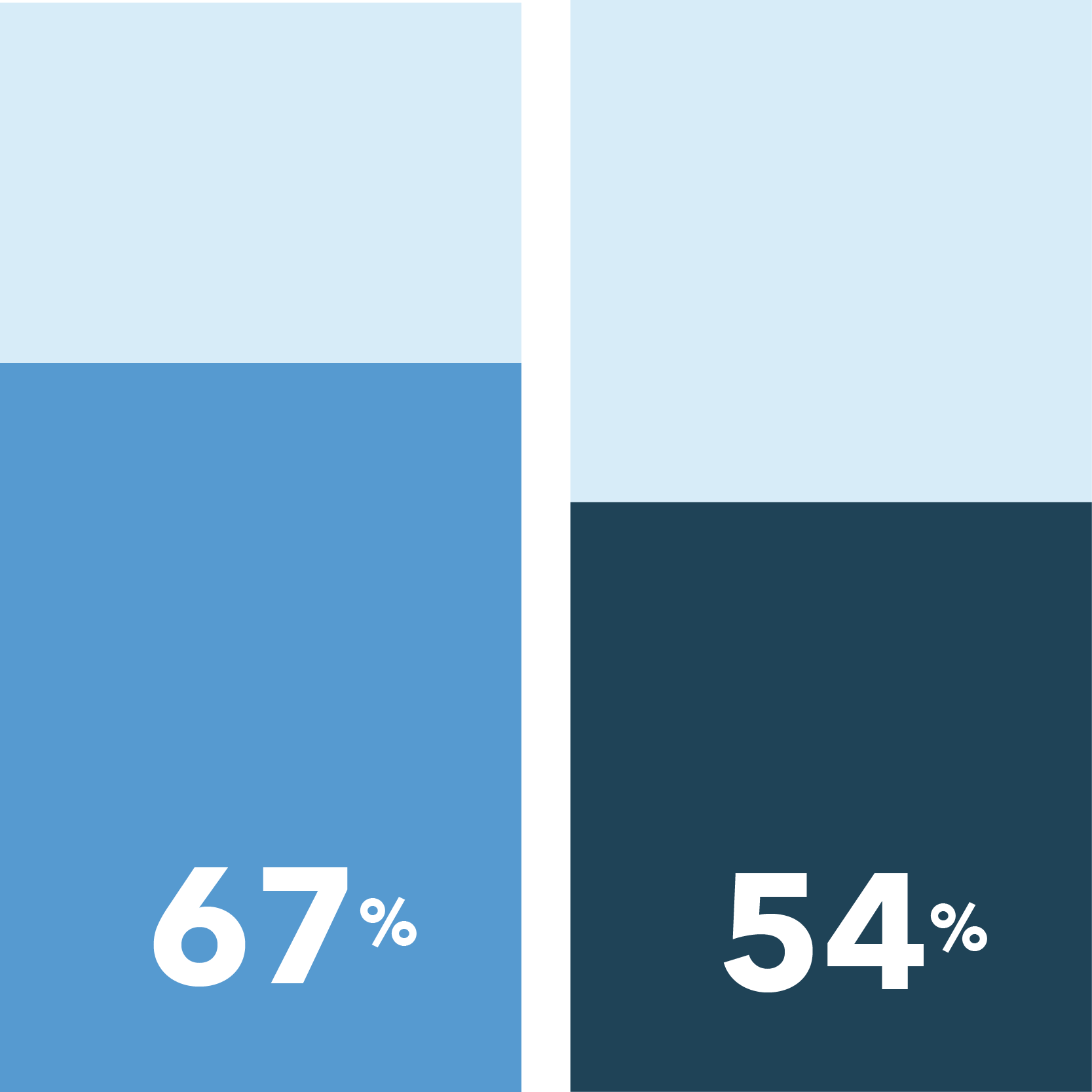
Ability to meet with a study doctor remotely
[/scorr_infographic]
[scorr_infographic width=”33″]
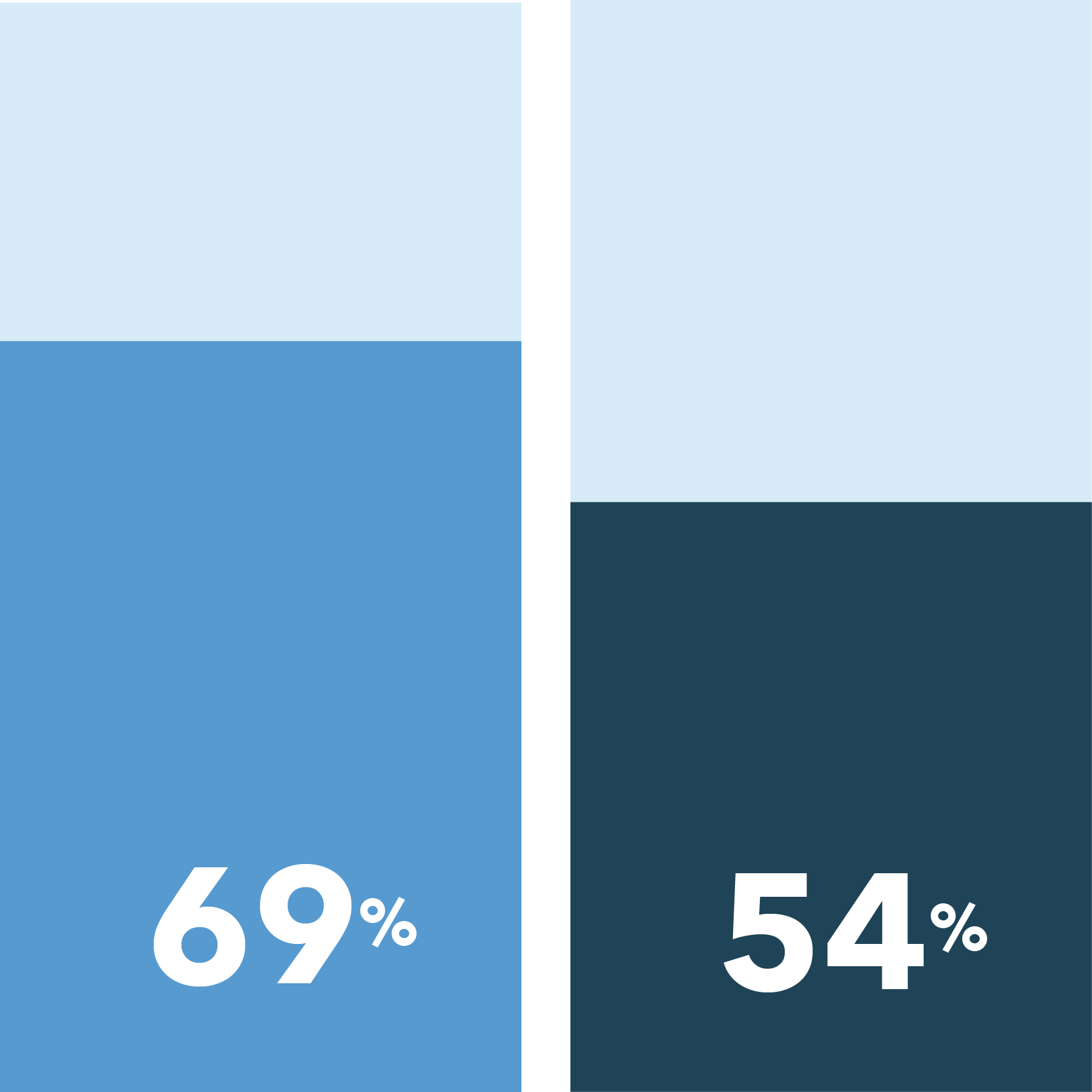
Home delivery of study medication
[/scorr_infographic]
[scorr_infographic width=”33″]
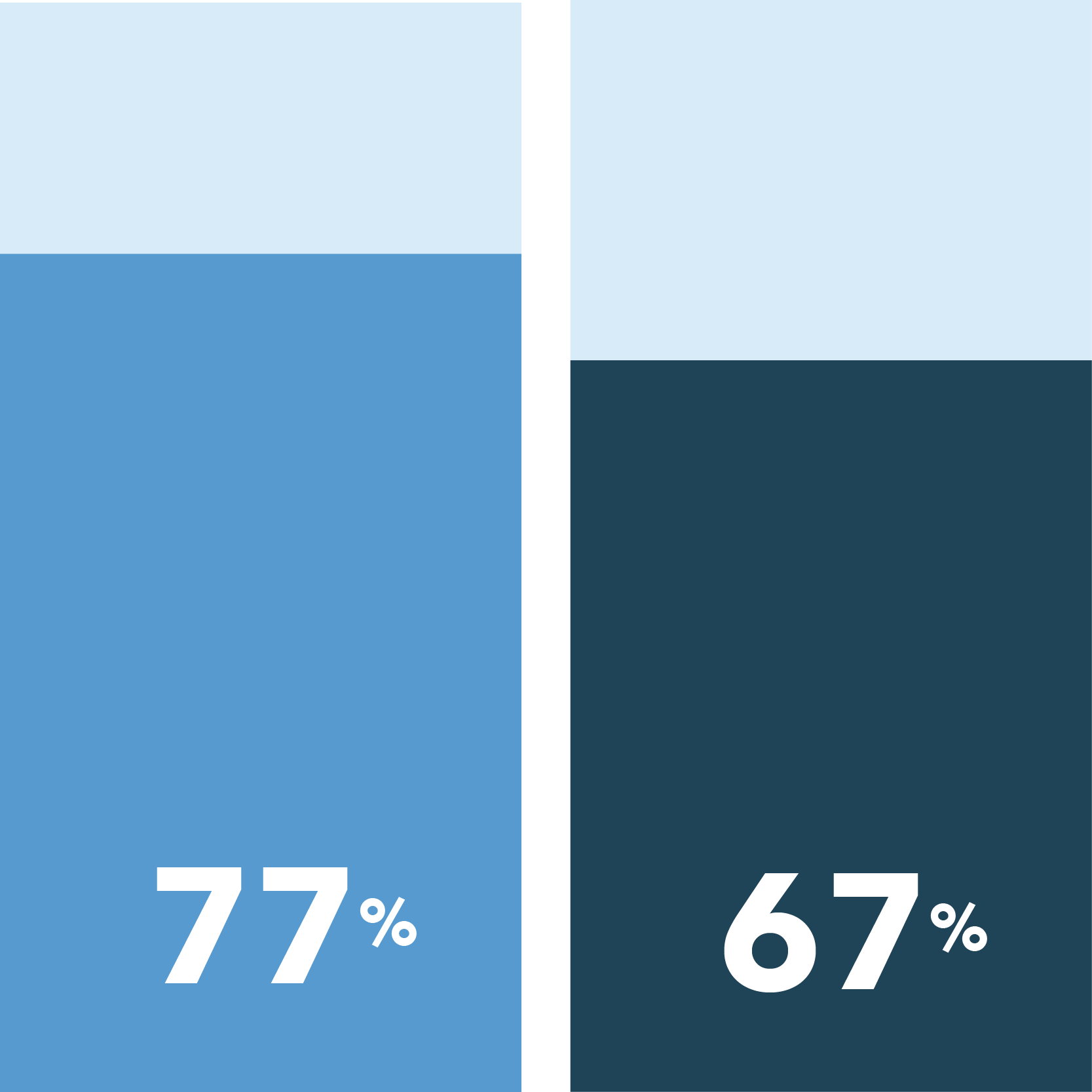
Health and safety protected at in-office visits
[/scorr_infographic]
[/scorr_infographic_wrap]
Clinical research, post-COVID-19.
If anything positive is to come out of this pandemic, it’s heightened clinical research awareness, and the opportunity for increased trust in our medical system. While concerns about COVID-19 exposure if enrolled in a trial continue to exist, there are precautions we as an industry can put in place to ease patient hesitation. Collectively, we have the opportunity to increase the level of trust in healthcare in general via the implementation of these patient-centric improvements to clinical trials.
We will continue to research the effects of the pandemic on clinical trials. Check back periodically for more updates on patient attitudes and sentiment toward clinical research.
Have any questions for our marketplace? Reach out by emailing covid19@subjectwell.com.





