Since the start of the pandemic, SubjectWell has kept a close eye on patient attitudes and feelings toward clinical trials. After all, right now is arguably the most critical time for clinical trial participation as COVID-19 cases continue to rise worldwide and vaccines and treatments are still being tested. By understanding what prevents or encourages patients to pursue clinical research, our industry can make sure patients feel at ease throughout the process, thus increasing involvement and, ultimately, developing better treatments.
For the fifth round of our ongoing survey, we polled 563 patients across the US between October 19 and 23, 2020, to understand how race influences patient concerns regarding clinical trial participation.
We also wanted to determine if respondents think clinical trial research is integral in finding a solution to the pandemic, and whether they feel that the US is conducting this research at a fast rate.
To learn more about our ongoing patient sentiment research, you can read our 2020 reports on round one of our survey (conducted March 19–23) here, round two (conducted April 2–8) here, round three (conducted May 7–13) here, and round four (conducted June 1–12) here.
What follows are some findings from our latest round of research.
Differences in COVID-19 concerns by race.
Regardless of race, most patients agree that clinical trials are an important step in finding a solution to the pandemic and are likely to participate in clinical research once COVID-19 no longer poses a threat. However, the data shows that there are still differences by race in patient concerns over the coronavirus and hesitations toward clinical trial participation within the current COVID-19 climate.
In the last round of our survey, note that we took a closer look at responses from individuals identifying as African American and Caucasian. However, in this fifth round of research, we compared the responses of individuals who self-identify as solely white, with the response of individuals who self-identify as American Indian, Asian, Black, Native Hawaiian, Pacific Islander, or multiracial (hereafter referred to as patients of color).
Patients likely to consider participating in a clinical trial for a medical condition other than COVID-19
[scorr_infographic_wrap aos=”1″]
[scorr_infographic width=”100″]
[scorr_bar_chart_single percent=”56″]
56% of Patients of color
[/scorr_infographic]
[scorr_infographic width=”100″]
[scorr_bar_chart_single percent=”65″]
65% of White patients
[/scorr_infographic]
[/scorr_infographic_wrap]
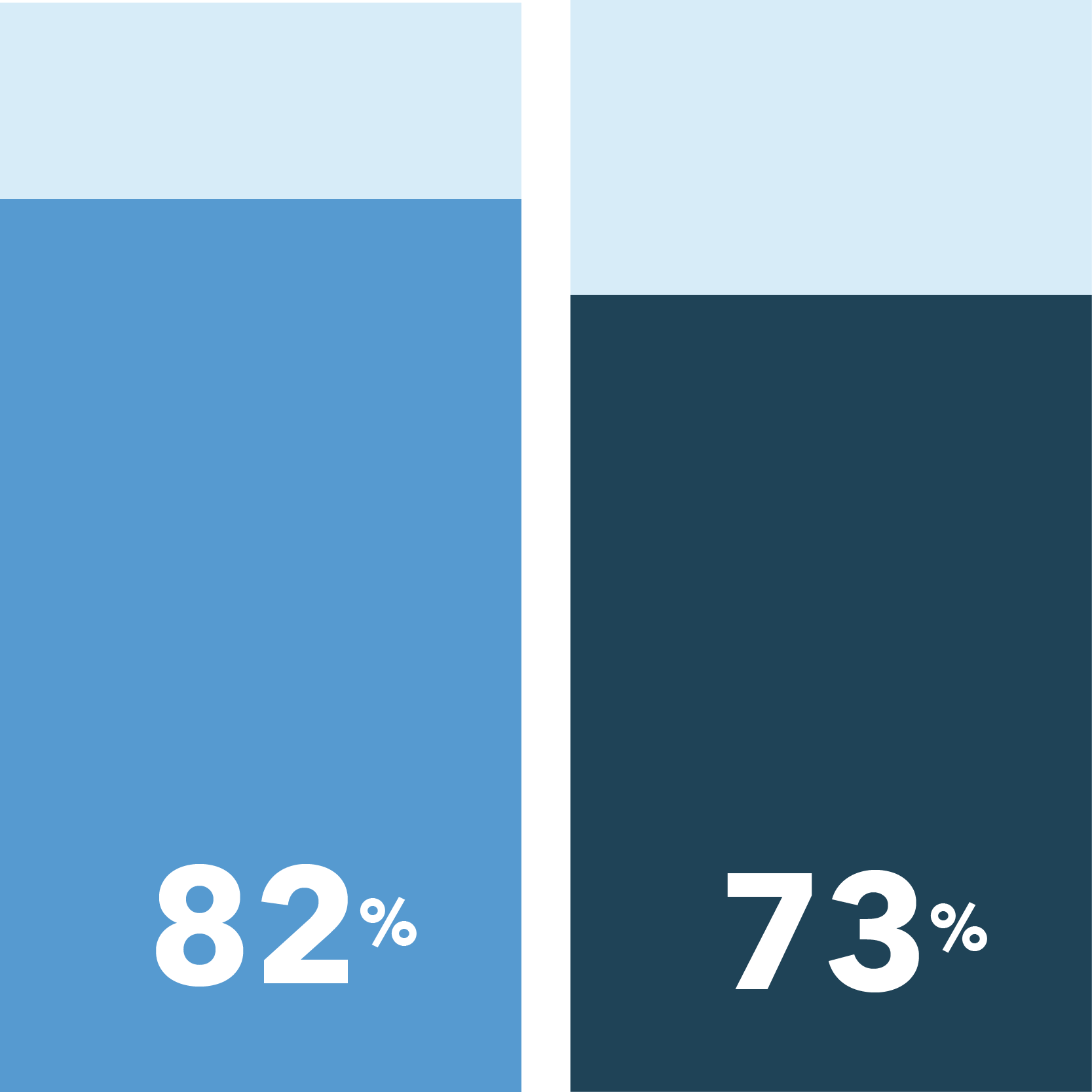
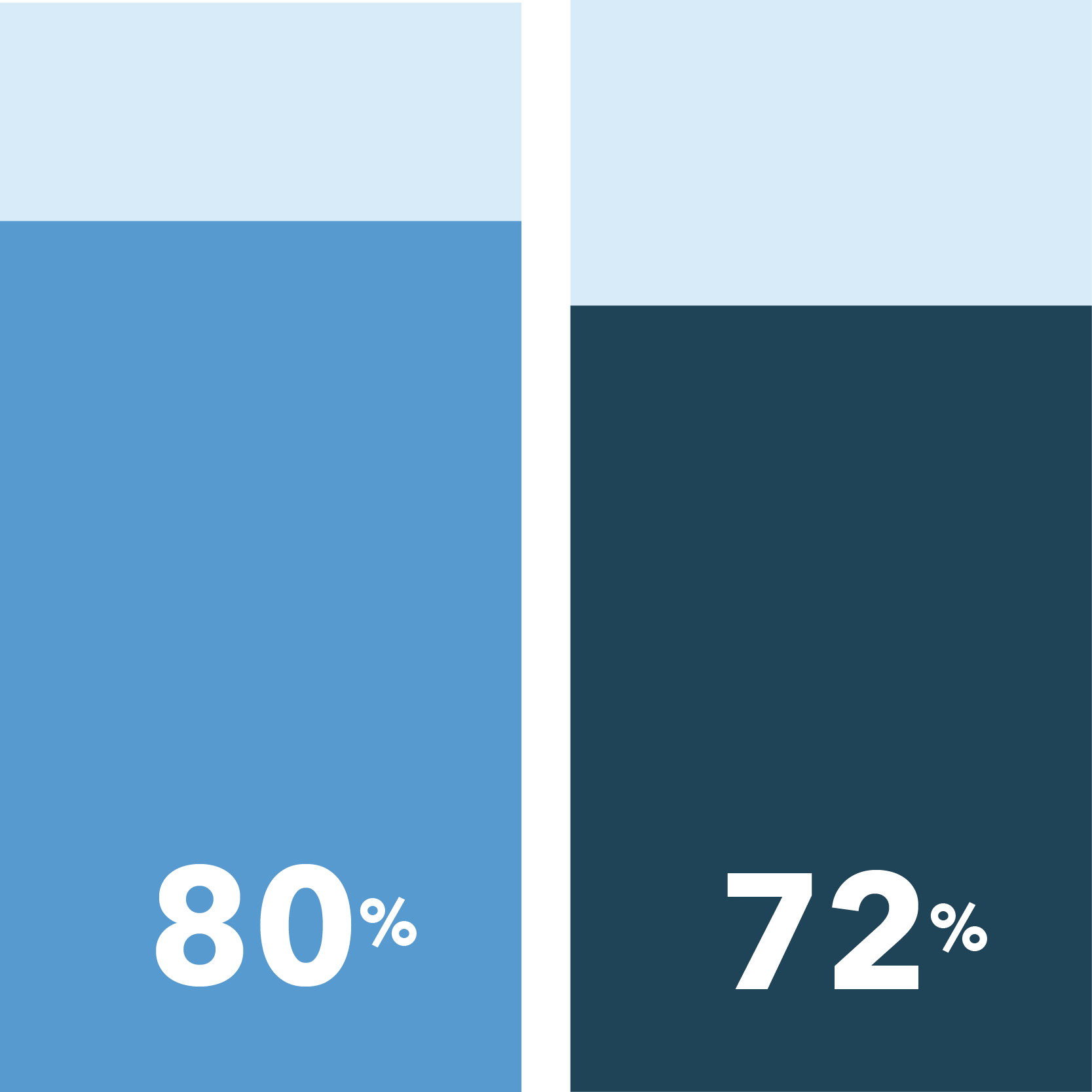
There are also differences when measuring concerns and reasons for clinical trial participation. Across the board, patients of color are more likely than white patients to rate various information and accommodations as important for soothing concerns about exposure to COVID-19 if they were to participate in non-COVID-19 clinical trials.
The following were identified as very important in easing COVID-19 concerns:
[scorr_infographic_key colors=”#649acb|#1f4357″ labels=”Patients of color|White patients”]
[scorr_infographic_wrap aos=”1″]
[scorr_infographic width=”25″]
Health and safety protected for in-person visits
[/scorr_infographic]
[scorr_infographic width=”25″]
Ability to communicate with study doctor remotely
[/scorr_infographic]
[scorr_infographic width=”25″]
Concierge services
[/scorr_infographic]
[scorr_infographic width=”25″]
At-home nurse visits
[/scorr_infographic]
[/scorr_infographic_wrap]
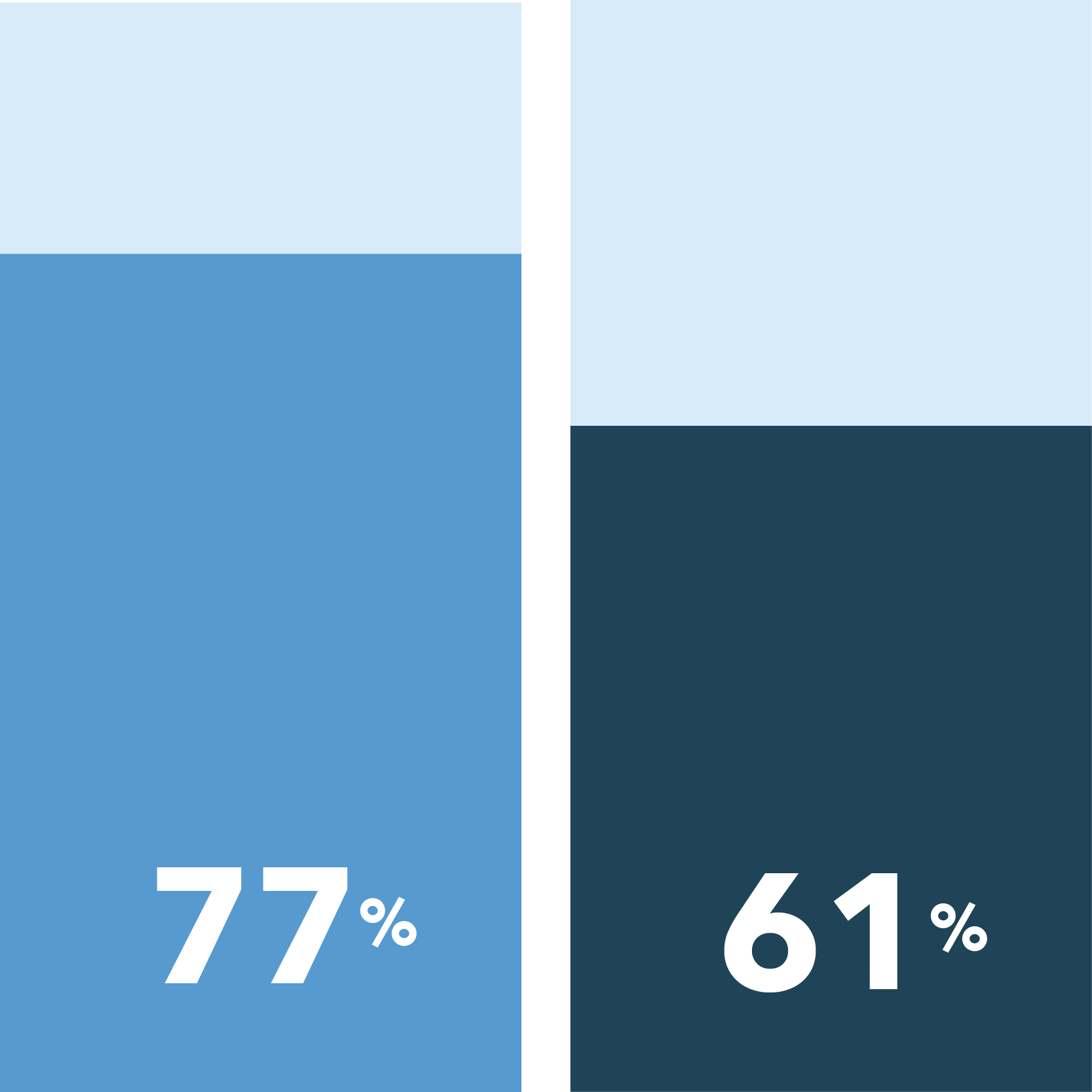
The following had been identified as important reasons to participate in clinical trials:
[scorr_infographic_key colors=”#649acb|#1f4357″ labels=”Patients of color|White patients”]
[scorr_infographic_wrap aos=”1″]
[scorr_infographic width=”25″]
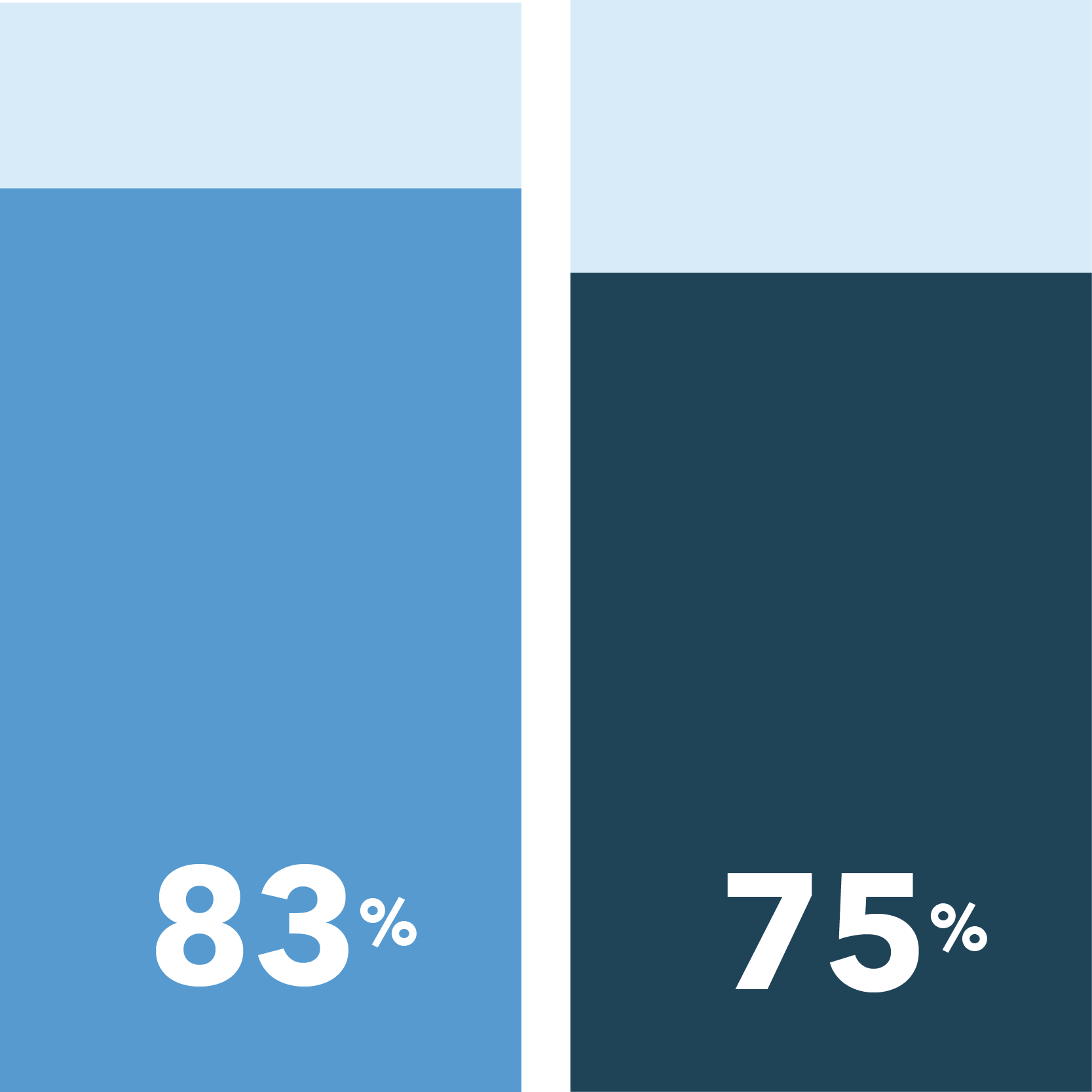
Obtain education about improving your health
[/scorr_infographic]
[scorr_infographic width=”25″]
Receive free medication and/or treatment
[/scorr_infographic]
[/scorr_infographic_wrap]
Additional overall findings.
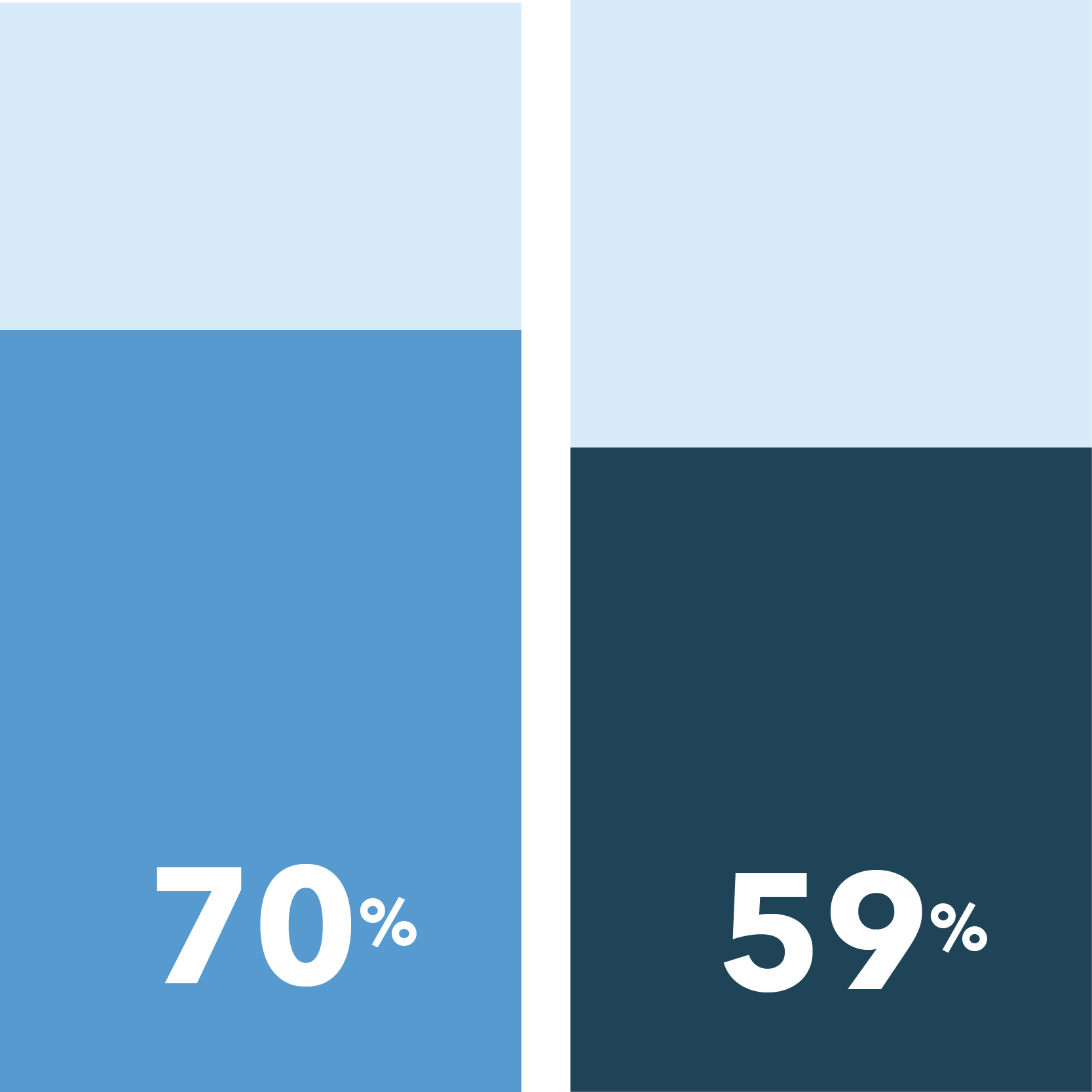
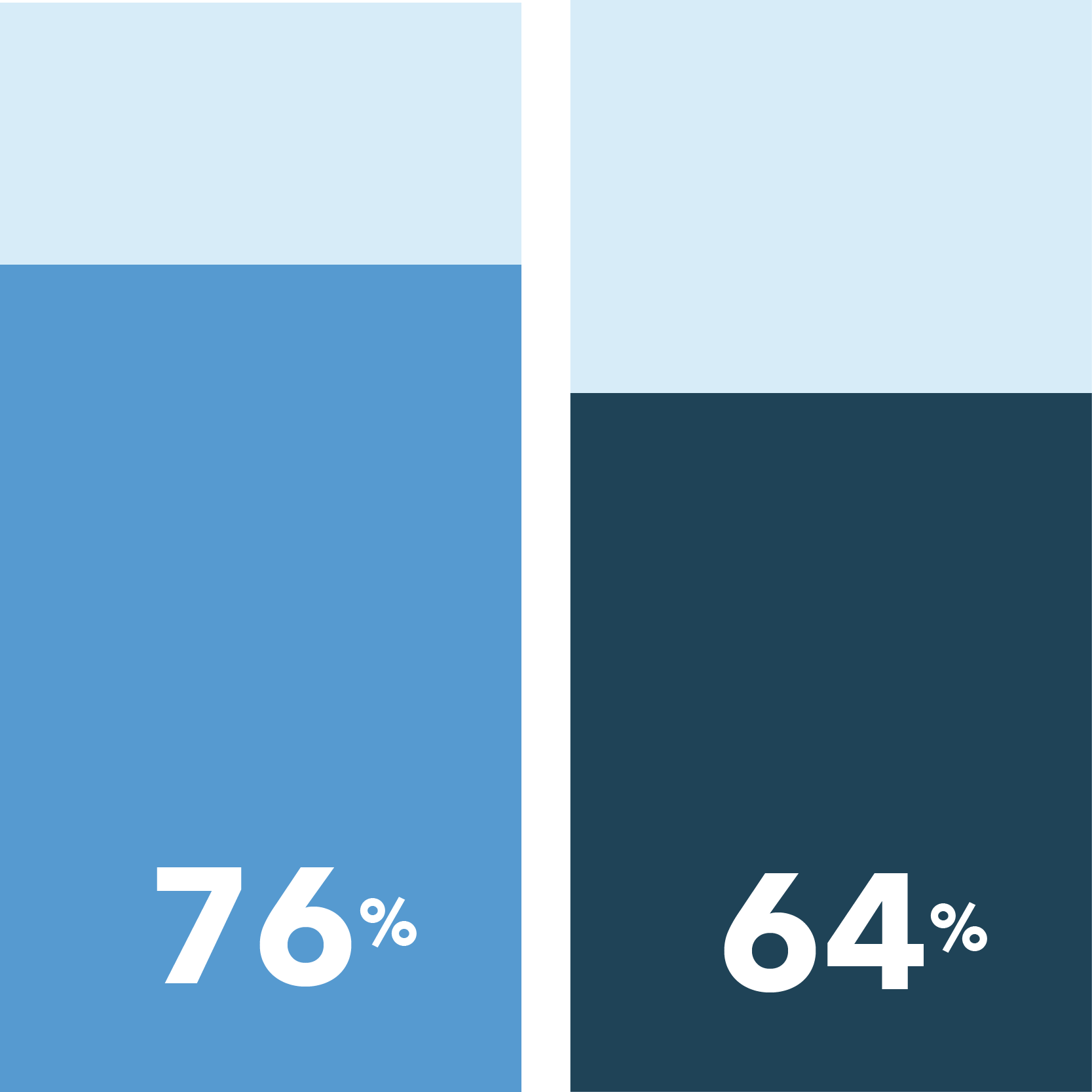
In this survey, 62% of patients responded they are likely to consider participating in a clinical trial for a condition other than COVID-19, a result in steady decline since the height of interest in May at 72%.
Patients considering participation in a clinical trial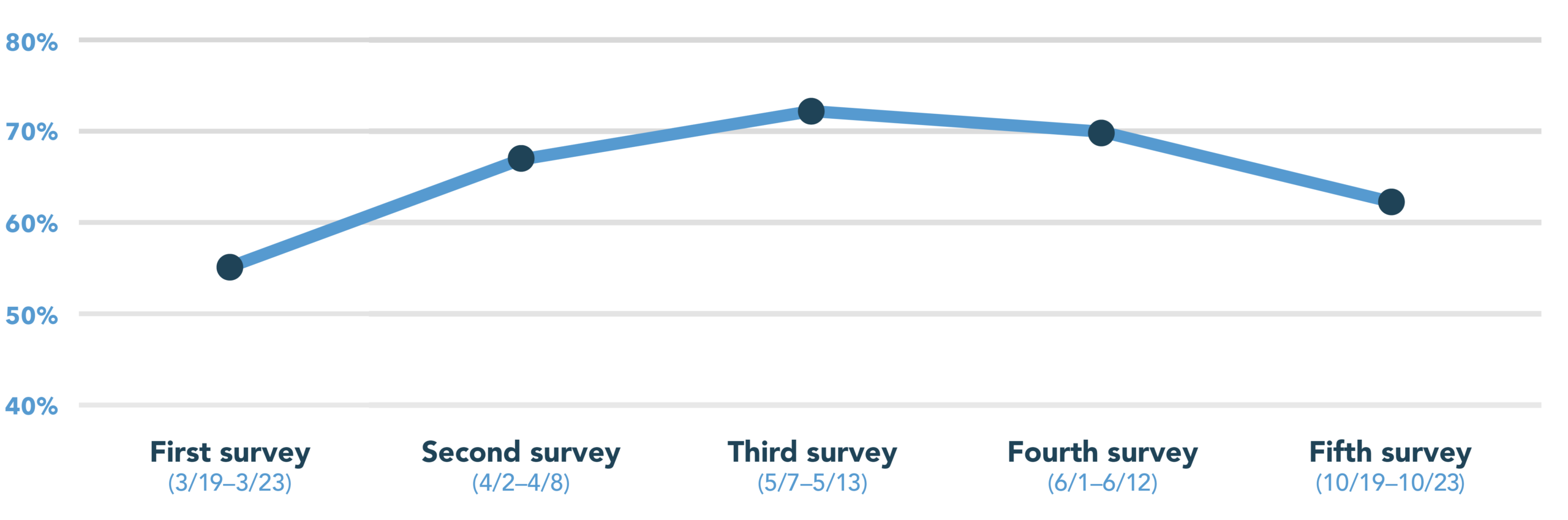
[scorr_infographic_wrap aos=”1″]
[scorr_infographic width=”100″]
[scorr_bar_chart_single percent=”89″]
89% of patients report that clinical research is important in finding a solution to the pandemic
[/scorr_infographic]
[scorr_infographic width=”100″]
[scorr_bar_chart_single percent=”43″]
Only 43% of patients report that they feel the US is conducting COVID-19 clinical trial research at a fast rate
[/scorr_infographic]
[scorr_infographic width=”100″]
[scorr_bar_chart_single percent=”70″]
70% of patients are likely to participate in a clinical trial if in-person visits aren’t required
[/scorr_infographic]
[scorr_infographic width=”100″]
[scorr_bar_chart_single percent=”76″]
76% of patients are likely to participate in a clinical trial after COVID-19 no longer poses a threat to public health
[/scorr_infographic]
[/scorr_infographic_wrap]
Moving forward with clinical research.
As the pharmaceutical industry continues to uncover potential solutions to the ongoing global health crisis, there is a responsibility to ensure patient safety and ease concerns as best as possible for those participating in clinical research. While we know differences in race affect areas of concern and reasons behind clinical trial participation, successful recruitment and retention efforts will need to adjust to ensure diversity in clinical trials. Our situation now presents the opportunity to better our practices, as well as our understanding of what’s needed to not only find the best treatment but also provide the best care for all those involved.
We will continue to research the effects of the pandemic on clinical trials as new developments unfold. Be sure to check back on our website for more updates on patient attitudes and sentiment toward clinical research.
Have any questions for our marketplace? Reach out by emailing covid19@subjectwell.com.