Background
A Top 10 pharmaceutical company partnered with SubjectWell to support patient recruitment for a chronic inducible urticaria (CIndU) clinical trial addressing a rare, dermatological condition. Initially, a large, internationally recognized agency managed media and creative, while SubjectWell provided the pre-screening website, call center, site management support, and performance reporting.
Challenge
The initial recruitment phase, managed by the external agency, struggled with low patient enrollment and high costs despite multiple attempts, including a creative refresh. SubjectWell identified that the agency’s approach required significantly improved performance and efficiency to meet recruitment targets within the stipulated timelines.
Strategy
SubjectWell took over the media management along with the down funnel management of the campaign, utilizing the pre-existing creative assets. Advanced digital optimization techniques across multiple platforms (social media and search) were deployed to enhance patient engagement and conversion rates.
Tactics
-
Comprehensive digital optimization across Facebook, Instagram, Snapchat,
Twitter, LinkedIn -
Re-targeting methods to maximize engagement
-
Precision targeting and strategic media placements to improve reach and conversion efficiency
SubjectWell drove 5x more randomizations and saved $1M in costs, dramatically outperforming a traditional agency in just a fraction of the time.
Results and funnel
SubjectWell’s digital media and targeting strategies consistently outperformed those of the traditional agency, leading to significant improvements in efficiency, cost savings, and patient enrollment outcomes.
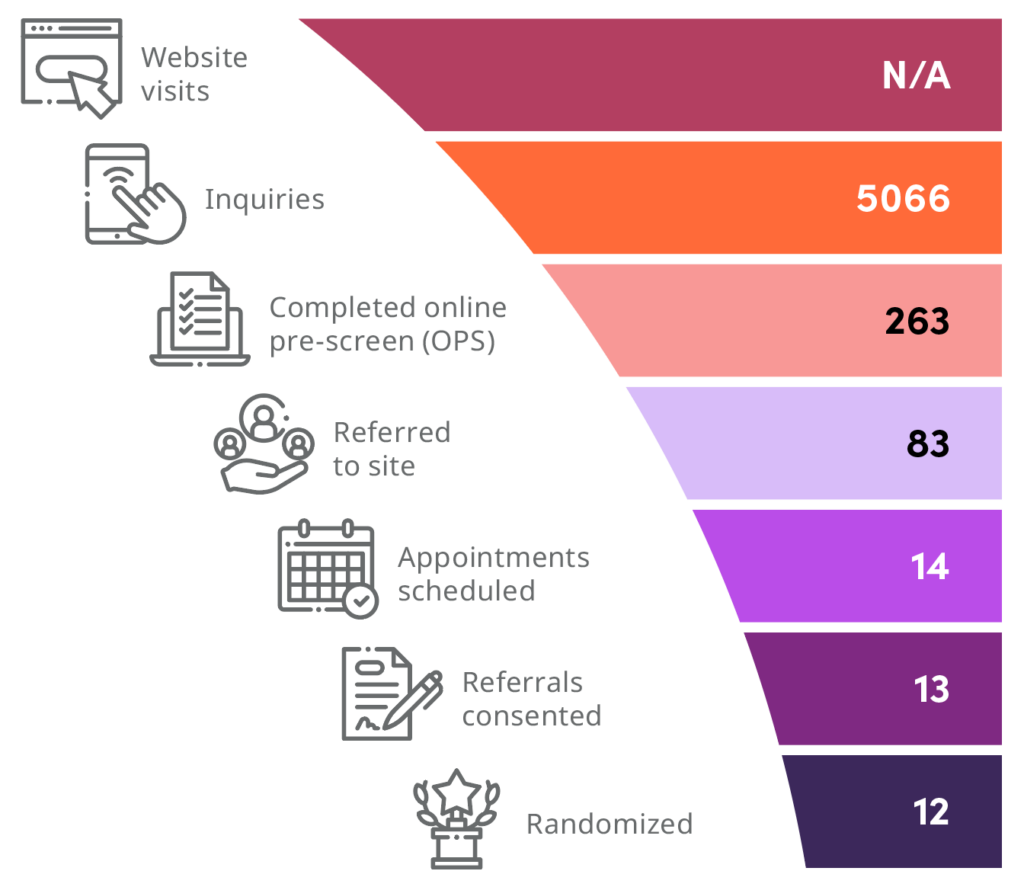
Key results
-
90% of US sites opted to receive referrals for the campaign
-
83% of patients were referred to the sites, with an OPS-to-referral conversation rate of 31.5%
-
Achieved a 92.8% conversion rate from appointment to consent, with a 7.7% screen fail rate
-
Achieved 13 randomizations, with a 14.5% referral-to-randomization rate
-
The campaign contributed to 14% of total US randomizations






