Reaching the unreachable

Case studies in depression and women’s health show how data, design, and digital recruitment uncovers overlooked patients, improves diversity, and accelerates trial timelines.
The missing piece in your patient recruitment platform

How real-time dashboards and instant access to medical records can reduce screening delays and improve enrollment efficiency.
Patient first, always

Why patient-centricity is the clearest path through uncertainty.
Accelerating patient-centric clinical trials

A four-part framework on how strategy, science, support, and creative can drive faster, more effective studies
Effectively recruiting patients online, including social media

A peer-reviewed, scientific approach to online recruitment in a depression study
Addressing stigma and logistical hurdles in psychiatric trials
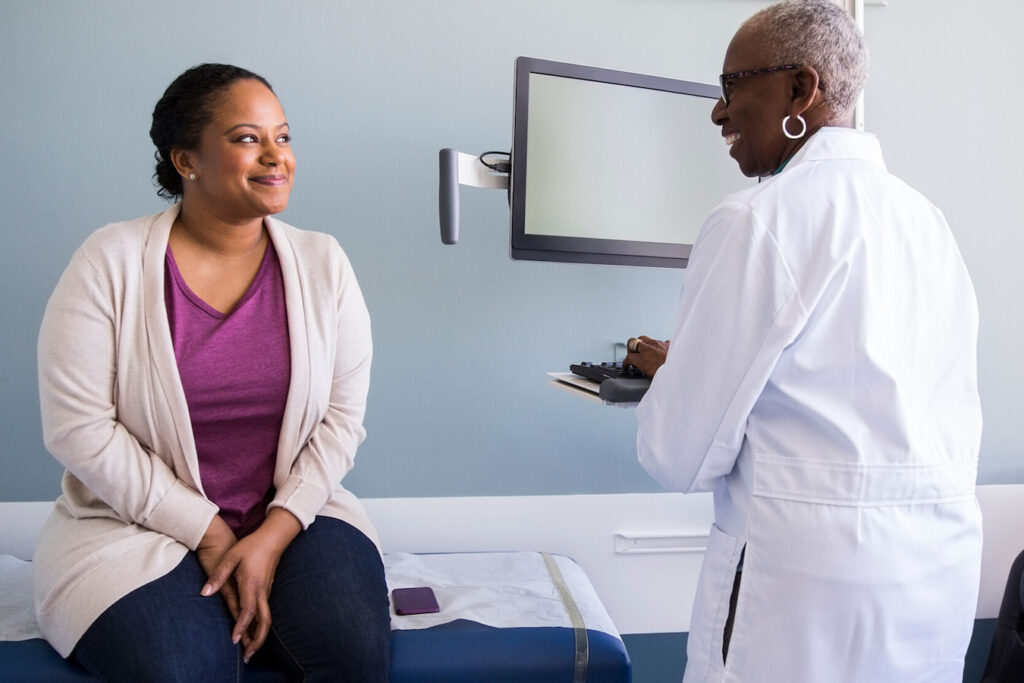
Learn how SubjectWell tackles key barriers in psychiatric trials—like stigma, documentation delays, and caregiver burden—with services that improve engagement, retention, and site efficiency.
New FMLA Guidance Empowers Patients to Participate in Clinical Trials

New FMLA guidance now protects job security for patients participating in clinical trials, removing a major barrier to accessing innovative treatments.
How to Prepare for FDA-Mandated Clinical Diversity Action Plans (DAPs)

Understanding FDA’s Guidance on Diversity Action Plans (DAPs) is vital for health equity in clinical trials. As DAPs become mandatory, sponsors must adapt recruitment strategies for inclusivity.
3 in 4 surveyed patients show strong interest in replacing medications with both approved and experimental treatments.

This growing openness offers a valuable opportunity for sponsors and CROs to engage motivated participants if recruitment strategies are aligned with patient motivations and trust.