-

Blog
SubjectWell + Clariness: Why this merger matters
4 min
●January 27, 2026
 Fred MartinChief Executive Officer
Fred MartinChief Executive Officer -

Blog
How to apply science to your patient protocol
6 min
●January 12, 2026
 Richard TownePrincipal Solutions Architect
Richard TownePrincipal Solutions Architect -

Blog
Clinical trials don’t take holidays
3 min
●December 15, 2025
 Richard TownePrincipal Solutions Architect
Richard TownePrincipal Solutions Architect -

-

Blog
Keeping clinical trial patients engaged during the holidays
3 min
●November 11, 2025
SubjectWell Team

How real-time dashboards and instant access to medical records can reduce screening delays and improve enrollment efficiency.
- CRO, Site Network, Sites, Sponsors
- API & Integrations, OneView with Instant Access Medical Records, Site Support

Why patient-centricity is the clearest path through uncertainty.
- CRO, Site Network, Sites, Sponsors
- Full-Site Support, OneView with Instant Access Medical Records, Patient Care & Trial Retention, Patient Companions, Patient Support, Site Support, Study Connect

A four-part framework on how strategy, science, support, and creative can drive faster, more effective studies
- CRO, Site Network, Sites, Sponsors
- Patient Care & Trial Retention, Patient Panels & Surveys, Patient Support, Site Support, Study support

A peer-reviewed, scientific approach to online recruitment in a depression study
- CRO, Site Network, Sites, Sponsors
- Digital Marketing, Study Connect, Study support
- Mental Health

In our Behind the Screens series we are spotlighting our Site Companions: the dedicated site engagement specialists, who power site support and clinical trial efficiency.
- Patient and Site Companions, Site Support

In this Behind the Screens spotlight, we feature Merusha Naidoo, a site team leader whose relationship-driven approach helps ease burden and boost performance across trial sites around the world.
- Patient and Site Companions, Site Support

SubjectWell CEO Fred Martin explores why symbolic support isn’t enough and how the steps the clinical trial industry needs to make to drive equity, representation, and better outcomes for LGBTQ+ individuals.
- CRO, Sponsors
- Patient Care & Trial Retention, Patient Support
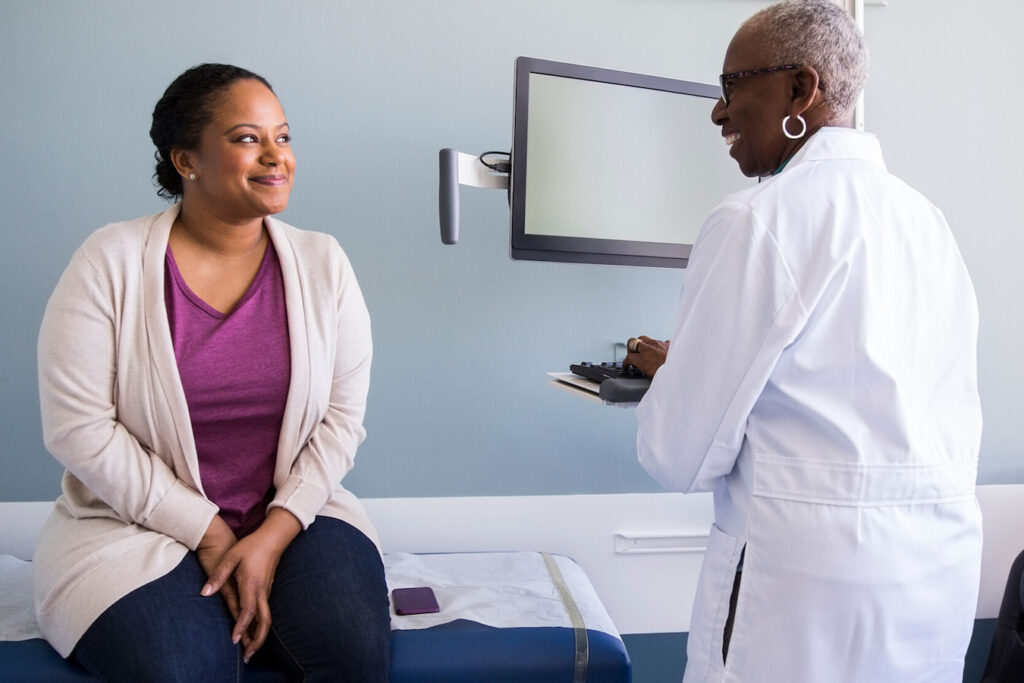
Learn how SubjectWell tackles key barriers in psychiatric trials—like stigma, documentation delays, and caregiver burden—with services that improve engagement, retention, and site efficiency.
- CRO, Site Network, Sites, Sponsors
- OneView with Instant Access Medical Records, Patient Care & Trial Retention, Patient Support, Secondary Screening, Site Support, Study support, Translations, Virtual Waiting Room
- Mental Health

Not every GLP-1 trial participant sees their condition in the same way. From diabetes to obesity and addiction, patient mindsets influence trial participation. Learn why tailored engagement strategies are essential for successful GLP-1 recruitment.
- CRO, Sponsors
- Full-Site Support, Patient and Site Companions, Patient Care & Trial Retention, Patient Companions, Patient Support, Secondary Screening, Site Support, Study support, Translations
WHY SUBJECTWELL
It’s time to transform patient recruitment
-
300,000
patients added monthly to our network
47%
registered patients of color
100+
clinically trained patient companions
-
300,000
patients added monthly to our network
47%
registered patients of color
100+
clinically trained patient companions
-
300,000
patients added monthly to our network
47%
registered patients of color
100+
clinically trained patient companions
Their tailored approach helped us meet our recruitment target ahead of schedule. Highly recommend!
Name
Job Description
Accelerate your recruitment today
SubjectWell is here to help you reach, recruit, and retain the patients you need for a successful clinical trial. Schedule a demo to see how SubjectWell+ can support your clinical study’s patient recruitment goals.