The link between site success and trial acceleration
Every sponsor wants to bring therapies to patients faster. But acceleration doesn’t come from bigger budgets or automation. It starts by guaranteeing that investigator sites are set up for success. When sites have the resources, staffing, and support to manage growing complexity, timelines shorten, data quality improves, and patient experience becomes a true differentiator.
As shared during our recent webinar, “Redefining Clinical Success for Sites Through Patient-Centered Support,” SubjectWell and Rovia Clinical Research explored how patient-first operations directly drive trial speed and predictability.
Why site support drives acceleration
Approximately 30% of patients drop out of clinical trials after randomization, with even higher rates in longer and more complex Phase 3 studies. Each day of delay can cost sponsors $600,000 to $8 million in lost revenue, while extended timelines can delay approvals by six to twelve months.
Richard Towne, Principal Solutions Architect at SubjectWell, summed it up:
“Every single dropout is something to be prevented. If you can do something to minimize these patients dropping out, it’s very important.”
When sites have the tools and staffing to stay ahead of complexity, that stability translates directly into faster, more predictable enrollment. As Stacia Wilson, Associate Director of Site Recruitment Support, explained:
“It’s not just the patient that needs support. Someone has to administer the exams, draw blood, collect data, and keep everything on track. The site carries that responsibility too.”
Measuring what matters
To help sponsors identify performance risks early, SubjectWell developed the Patient Burden Index and Site Burden Index. These data-driven tools quantify operational and participant efforts. By measuring the right inputs, sponsors can make informed design and resourcing decisions that improve recruitment efficiency and site throughout.
Richard Towne noted:
“It’s thinking carefully about what’s scientifically necessary versus what’s just nice to have.”
And for Samantha Schmidt, Clinical Research Recruitment Manager at Rovia Clinical Research, continuous feedback is key:
“We send monthly patient satisfaction surveys to actively enrolled patients to understand their experience. That feedback helps us make data-driven adjustments, whether that’s improving scheduling, communication, or comfort during visits.”
These insights enable sponsors to design protocols and partnerships that support site success and accelerate their studies in turn.
The acceleration equation: retention = predictability
Retention is the foundational metric for predictable timelines and data quality.
“When someone is helping support a participant directly, they help maintain compliance, reduce missed visits, and enable faster database locks,” said Rich.
Through Full-Site Support, SubjectWell’s staff-augmentation service, Patient Companions and Site Companions guide participants through every stage of their journey to keep engagement high and operations steady.
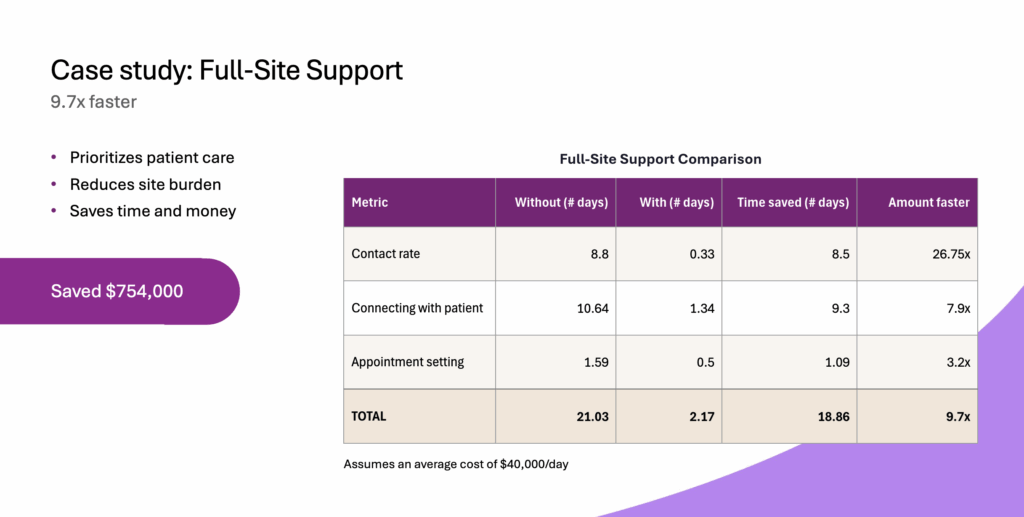
The impact:
- Contact rates improved 25.7%
- Appointments scheduled 3.2× faster
- Time-to-randomization reduced by 38%
- 71% of U.S. randomizations in a pediatric peanut-allergy study achieved at a 96% lower cost per referral than the previous vendor
As Stacia noted:
“Full-Site Support services can make all the difference. They help teams manage large patient volumes efficiently while still maintaining quality interactions.”
Retention and acceleration are two sides of the same result: predictable performance.
Technology that connects, not complicates
Technology should simplify, not slow down, site operations. Many coordinators still juggle multiple sponsor systems, duplicating work, and wasting time.
Samantha summarized the reality:
“The biggest challenge is the sheer number of systems. Each sponsor seems to have their own portal, and staff spend hours entering the same information multiple times.”
To solve that, Stacia introduced OneView, SubjectWell’s unified portal:
“OneView brings everything together in one place per site. You can view all patients and their recruitment journey in a single view, including medical history, upcoming visits, and reminders—all integrated. It lets sites focus on patient care.”
Because when data and communication flow seamlessly, studies do too.
Partnership is the ultimate accelerator
Acceleration thrives when sponsors and sites operate as partners, not silos.
As Samantha shared:
“Staffing and retention are constant challenges. High-performing coordinators are often rewarded with more work. It’s our responsibility to check in, listen to feedback, and make workloads sustainable.”
That culture of partnership mirrors SubjectWell’s philosophy: when sites feel supported, sponsors gain confidence and predictability.
As Rich concluded during the Q&A:
“To accelerate a trial, you need to ensure that patients are qualified and motivated—and that sites aren’t overwhelmed. When you focus on those fundamentals, acceleration happens naturally.”
The takeaway
To accelerate a trial, guarantee site success. To guarantee site success, invest in integrated services that enable retention, consistency, and predictability. When patients feel guided and sites feel equipped, sponsors complete studies better and faster.
Because at the end of the day, the fastest path to trial completion is the human one.






